| |

January 27, 2026: USPTO Weather-Related Closing Due to inclement weather, the USPTO was officially closed on Monday, January 26, and Tuesday, January 27, 2026. The USPTO considers these dates federal holidays. See USPTO Status page. Per 35 USC 21, where a 3-month 1.704(b) applicant response deadline falls on either date, the effective 3-month deadline for determining any PTA reduction is extended until Wednesday, January 28, 2026, which was the next business day the USPTO was open. See ArQule v. Kappos, 793 F.Supp.2d 214 (D.D.C. 2011); MPEP § 2732. We have updated our calculator to automatically consider this closing (along with other federal holidays) in analyzing PTA rules when the Apply ArQule v. Kappos option (on by default) is selected on the Apply Term Rules tab. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
June 30, 2025: USPTO Implements Monetary Fine and PTA Penalty for False Entity Status Claims The USPTO has implemented a monetary fine and PTA penalty for applicants making false assertions or certifications concerning small or micro entity status. At its discretion, the USPTO may review entity status claims for compliance with relevant rules. If it makes a preliminary determination that a false assertion or certification resulted in a fee underpayment, the USPTO will issue a combined notice and order setting forth the basis for its finding and providing an opportunity for the applicant to respond. Thereafter, the USPTO will generally issue a final determination, based on the record as a whole, if a false claim resulted in a fee underpayment and the fine amount, if any, being assessed. The fine will be not less than three times the amount an entity failed to appropriately pay, unless the entity can show its false assertion or certification was made in good faith. Concerning PTA, the Notice provides: In the event that the USPTO makes a final determination that the application contains a false assertion or certification that resulted in the payment of at least one fee in an unentitled reduced amount, there will be a patent term adjustment (PTA) impact for the application. Under 37 CFR 1.704(c), circumstances that constitute a failure of the applicant to engage in reasonable efforts to conclude processing or examination of an application may result in the reduction of the period of adjustment set forth in 37 CFR 1.703. There will be a delay in prosecution corresponding to the USPTO removing an application from examination, pending the resolution of the false assertion or certification in the application. Because the delay is the result of a false assertion or certification, the delay is a failure to engage in reasonable efforts to conclude processing or examination of the application, starting on the date the USPTO issues the combined notice and order and ending on the date all appropriate fee deficiencies and any assessed fine are paid in full. The above overview is general in nature. Please see the USPTO Notice for complete details. If you have questions, please contact us by email to Support@PatentTerm.com.
May 29, 2025: Silver Anniversary of AIPA Patent Term Adjustment To restore fairness and proper incentives to inventors, the American Inventors Protection Act (AIPA) was enacted in 1999 to compensate for Patent Office examination delays. AIPA guaranteed that diligent applicants would always receive a patent term of no less than 17 years, sometimes considerably more, up to 20 years. Today marks the 25th anniversary of the effective date of AIPA Patent Term Adjustment. Original patent applications became subject to AIPA Patent Term Adjustment based on a filing date on or after May 29, 2000.
September 28, 2024: USPTO Coding Glitch Causes PTA Calculation Errors The USPTO announced that a programming error in a recent software update caused its PTA calculation algorithm to generate incorrect PTA's for some patents issued from March 19, 2024 to July 30, 2024. Specifically, the algorithm miscalculated: The USPTO believes other parts of its PTA calculation under 35 USC 154(b) were not impacted by the error, and that its computer algorithm is now corrected. The USPTO contends that only approximately 1% of patents during this period ended up with an incorrect PTA. For many other patents, the inaccurate amount of “A” delay was offset by the inaccurate amount of “Overlap”, so the overall amount of PTA ended up being correct. The USPTO will not sua sponte correct patents affected by this error. Instead, patentees seeking a revised PTA determination based on the error must submit a timely request for reconsideration of PTA under 37 CFR 1.705(b). The deadline to file this request is no later than two months from the patent grant date, extendible by an additional 5 months per 37 CFR 1.136(a). The USPTO Office of Petitions will manually review each request filed under 37 CFR 1.705(b). The USPTO will waive both the 37 CFR 1.705(b)(1) request fee (37 CFR 1.18(e)) and the 37 CFR 1.136(a) extension fee where the sole reason for contesting the PTA is calculation error in the amount of “A” delay and "Overlap". Accordingly, the 1.705(b) request can be filed without a fee within seven months of the patent grant date. The USPTO states that the patentee should mention the Notice when seeking waiver of the fees, and patentee may consider authorizing fees to be charged to a deposit account if the waiver is not applicable. For previous requests, fees already paid cannot be refunded. Patentees are advised to carefully check USPTO-calculated PTA in light of this error and seek correction in appropriate cases. The above overview is general in nature. Please see the USPTO Notice for complete details. If you have questions or would like help determining if your patent is affected, please contact us by email to Support@PatentTerm.com.
August 13, 2024: Federal Circuit Narrows 2023 Cellect ODP/PTA Decision, Allowing Some Parent Patents to Keep PTA In an important decision concerning the relationship between obviousness-type double patenting (ODP) and PTA, Allergan v. MSN Labs (Fed. Cir. 2024), the US Court of Appeals for the Federal Circuit clarified the application of its 2023 Cellect decision in a common circumstance affecting patent families. Specifically, the Court held that "a first-filed, first-issued, later-expiring claim cannot be invalidated [via ODP] by a later-filed, later-issued, earlier-expiring reference claim having a common priority date." (emphasis added.) This holding provides some relief for many patent families, where first-filed, first-issued parent applications often experience significant USPTO examination delays, resulting in more PTA than later-issued child applications. This ruling means more patentees will be able to keep the parent's PTA, rather than disclaiming it to avoid ODP over a later-filed, later-issued, earlier-expiring child. Citing "the purpose of the ODP doctrine, which is to prevent patentees from obtaining a second patent on a patentably indistinct invention to effectively extend the life of a first patent," the Court held that a later-filed, later-issued, earlier-expiring child patent logically does not extend the later-expiring parent's coverage: [I]n many ways this case is “a prime example” of when ODP does not apply. See id. When seeking patent protection, it is not atypical for a patent applicant to first seek to protect the most valuable inventive asset (e.g., a pharmaceutical genus claim) before filing continuing applications on enhancements or modifications to that inventive asset (e.g., a particular compound in that genus, a method of using the compounds of that genus, etc.). And it is unsurprising that prosecution of a first-of-its-kind invention can be protracted, requiring greater time and effort by the applicant and examiner alike, such that any eventual patent on that invention is awarded some amount of PTA. Nor is it surprising that, for one reason or another (e.g., the examiner’s newfound familiarity with the subject matter), a subsequently filed continuing application claiming the same priority date and covering a modification of that invention proceeds much more efficiently through prosecution such that any patent awarded to that modification receives little to no award of PTA. As a result, that later-filed, later-issued continuing, or “child,” patent, whether subject to a terminal disclaimer over the parent or not, generally expires no later than the parent patent. That child patent does not, then, result in any extension of patent term of the invention claimed in the parent patent given that it expires first. Nor can the parent patent be said to result in an extension of patent term of the invention claimed in the child patent when, as here, the claims in the child patent did not even exist until after the parent patent issued. (emphasis added.) The Court further held that in these circumstances, requiring a patentee to disclaim the parent's PTA to avoid ODP, would deny the patentee the term compensation guaranteed by the American Inventors Protection Act (AIPA): To hold otherwise—that a first-filed, first-issued parent patent having duly received PTA can be invalidated by a later-filed, later-issued child patent with less, if any, PTA—would not only run afoul of the fundamental purposes of ODP, but effectively abrogate the benefit Congress intended to bestow on patentees when codifying PTA. That is because such a holding would require patent owners, in order to preserve the validity of the parent patent, to file a terminal disclaimer disclaiming any term of the parent that extends beyond that of the child, which, given that the patents share a priority date, would amount to the disclaimer of only PTA. That parent patent, then, would not receive the benefit of its congressionally guaranteed patent term, see 35 USC § 154(b), and would instead be limited to the, presumably shorter, term of its own child. Such a result would be untenable. Note that a Petition for Certiorari is pending in the Cellect case, which may be affected by this ruling. Given the importance of this issue, we expect substantial commentary from the patent community, e.g.: - Dennis Crouch, Family Planning Patent Style: Allergan, Cellect, and the ODP Maze, Patently-O (Aug. 13, 2024)
- Eileen McDermott, CAFC Says ‘First-Filed, First-Issued, Later Expiring Claim’ is Not Invalid for ODP, IPWatchdog (Aug. 13, 2024)
Applicants are advised to carefully consider the Cellect and Allergan Opinions and related legal commentary when prosecuting interrelated patent families. The above overview is general in nature. Please see the Federal Circuit Opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
July 19, 2024: EDVA Upholds USPTO PTA Reduction for Applicant Delay in Correcting Shading In Lundbeck v. USPTO, 1:23-cv-1105 (E.D. Va. 2024), the US District Court for the Eastern District of Virginia granted summary judgment upholding the USPTO's PTA reduction for applicant's failure to place its application in "Condition for Examination" within 8 months of national stage commencement. Specifically, applicant's original specification contained shaded tables, later deemed by the USPTO to lack sufficient clarity and contrast for scanning, which was not corrected by applicant until almost 13 months from national stage commencement. 35 USC 154(b)(2)(C) mandates that applicant delay be subtracted from USPTO delay to compute PTA, and that the USPTO "shall prescribe regulations establishing the circumstances that constitute a failure of an applicant to engage in reasonable efforts to conclude processing or examination of an application." One such prescribed delay is the applicant taking more than 8 months to place an application in "Condition for Examination". 37 CFR 1.704(c)(13) provides: Circumstances that constitute a failure of the applicant to engage in reasonable efforts to conclude processing or examination of an application also include the following circumstances, which will result in the following reduction of the period of adjustment set forth in § 1.703 to the extent that the periods are not overlapping: * * * (13) Failure to provide an application in condition for examination as defined in paragraph (f) of this section within eight months from either the date on which the application was filed under 35 USC 111(a) or the date of commencement of the national stage under 35 USC 371(b) or (f) in an international application, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the day after the date that is eight months from either the date on which the application was filed under 35 USC 111(a) or the date of commencement of the national stage under 35 USC 371(b) or (f) in an international application and ending on the date the application is in condition for examination as defined in paragraph (f) of this section; and 37 CFR 1.704(f) sets forth a myriad of requirements to meet "Condition for Examination" including the need for "papers in compliance with § 1.52". In the subject prosecution, about 10 months after national stage commencement, the USPTO first notified Lundbeck that it failed to meet Rule 1.52 via a Notice to File Corrected Application Papers: The application is not in compliance with 37 CFR 1.52 and PCT Rule 11 because pages 80-84, AND 139 CONTAIN(S) SHADING IN THE TABLE(S). Application papers (including any electronically submitted papers) must be presented in a form having sufficient clarity and contrast between the background of the paper and the writing thereon to permit the Office to electronically reproduce the papers by use of digital imaging and optical character recognition. See 37 CFR 1.52(a)(1)(v) and PCT Rules 11.2(a) and 11.9(d). Lundbeck argued that the USPTO's PTA reduction was improper because inter alia: (1) most of the delay was caused by the USPTO's 10-month delay in identifying the alleged defect; (2) the applicant responded to the Notice within 3 months as required by Rule 1.704(b); (3) the USPTO's application of Rule 1.704(c)(13) was arbitrary as it was not applied to other applications with nearly identical shading; and (4) the text in the shaded tables was in fact sufficiently clear and readily legible, and the USPTO failed to present substantial evidence that the gray shading turned the text illegible after electronic reproduction. The Court rejected these arguments, holding that the 37 CFR 1.704(c)(13) PTA reduction was not improper, as it falls to the applicant to submit a conforming specification: [P]rior to receiving the March 11, 2020 Formalities Letter, Plaintiff was put on notice, via 37 CFR § 1.52 and MPEP § 608.01(1), that the use of gray shading in the patent specification could impact legibility of the application's text and prevent the application from being in condition for examination. * * * Had Plaintiff received earlier notice from the USPTO of the shading issue, Plaintiff may have been able to fix the problem before the expiration of Rule 1.704(c)(13)'s eight month grace period. However, Plaintiff chose to submit a specification that included gray shading despite being on notice, vis-a-vis publicly available agency guidance, that such shading could affect legibility and prevent the application from being in condition for examination. In accordance with its statutory authority, the USPTO determined that Plaintiffs failure to provide the specification in condition for examination constitutes a failure to engage in reasonable efforts that warranted a reduction of the PTA calculation. The Court finds this to be consistent with the statute. * * * The USPTO consistently explained to Plaintiff that the agency believed the '047 Application was not in compliance with Rule 1.52(a)(1)(v), which is incorporated by reference into Rule 1.704(f), because of the gray shading in the tables on pages 80-84 and 139 of the Application. As discussed in depth above, guidance from the USPTO explains thoroughly the agency's concern with the issue of gray shading, and specifically, how it may impact legibility. Therefore, it was reasonable, and not a clear error of judgment, for the USPTO to determine that the gray shading could impact the legibility of the text and find that the application was not in condition for examination. Accordingly, the Court does not find that the agency's decision violates the APA. Slip Op. at 15 et seq. The Court also rejected Lundbeck's argument that the USPTO arbitrarily applied Rule 1.704(c)(13), because the USPTO provided examples of applying the PTA reduction in other similar cases, and because Lundbeck waived this argument by not first raising it during the administrative proceeding. Slip Op. at 22, 23. The above overview is general in nature. Please see the District Court Opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
August 31, 2023: Federal Circuit Affirms Double Patenting Invalidation of Patents with PTA In a decision potentially affecting many patent families, In re: Cellect, LLC (Fed. Cir. 2023), the US Court of Appeals for the Federal Circuit upheld a USPTO Patent Trial and Appeal Board’s (PTAB) determination that certain claims of four interrelated patents are unpatentable for obviousness-type double patenting (ODP). The Court held that ODP analysis must be based on the expiration date of each patent after its individual PTA has been added. For patent families subject to ODP, this holding may effectively negate PTA for USPTO examination delays granted by the American Inventors Protection Act, since patent term may be limited to the least delayed application in the family where a terminal disclaimer (TD) is required to overcome ODP. The four Cellect patents on appeal are part of a larger family. Because each claims priority from a common application, each patent would have expired on the same day but for individual grants of PTA for USPTO examination delays in each patent’s individual prosecution: 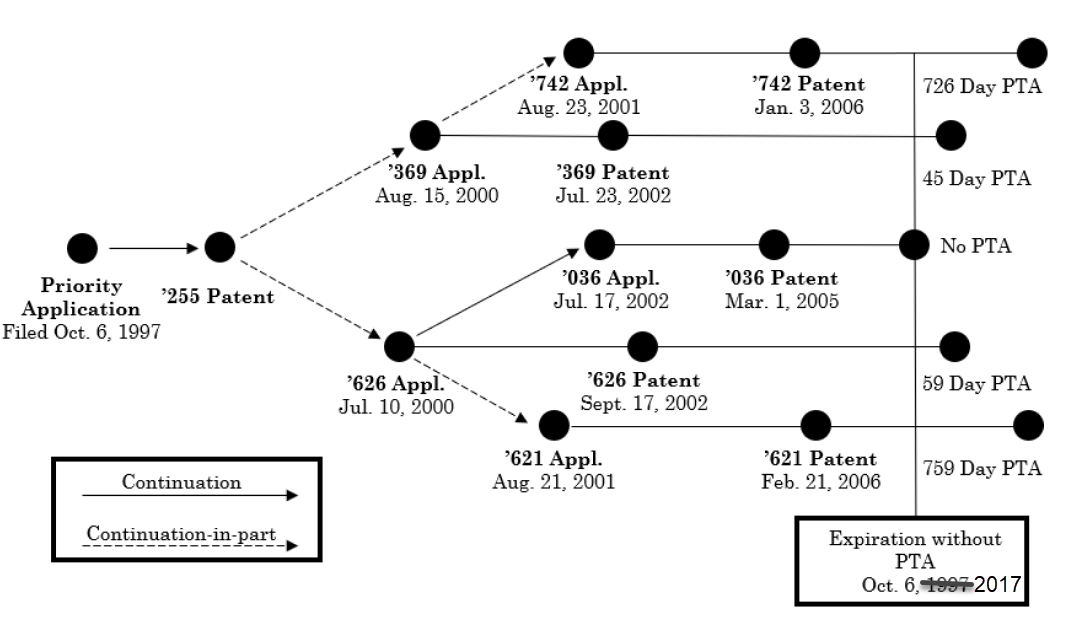 Several patents in the family had lengthy examination delays with PTA’s exceeding 700 days. The ODP invalidation, however, can be traced back to the ’036 patent, which did not receive any PTA, and thus retained its base expiration date twenty years after the filing of the priority patent application. Because of PTA, each of the invalidated patents had a later expiration date. Upholding the PTAB’s ODP invalidation, the Court held that ODP must be based on the expiration date of each patent after its PTA has been added: ODP is a judicially created doctrine that has its roots in 35 USC § 101, which states that an inventor may obtain “a patent” (i.e., a single patent) for an invention. ODP is intended to prevent a patentee from obtaining a time-wise extension of patent for the same invention or an obvious modification thereof and prevents an inventor from claiming a second patent for claims that are not patentably distinct from the claims of a first patent. A crucial purpose of ODP is to prevent an inventor from securing a second, later-expiring patent for non-distinct claims. This purpose applies equally to situations in which the later patents have received grants of PTA resulting from examination delays at the USPTO. Slip Op. at 16 (citations and internal quotations omitted). The Court distinguished prior decisions regarding 35 USC 156 Patent Term Extension (PTE) (term restoration for regulatory approval), which held that ODP is based on the expiration date before PTE has been added: The PTE and PTA statutes have quite distinct purposes. PTE is designed to effectively extend the overall patent term for a single invention due to regulatory delays in product approval. PTA is designed to extend the term of a particular patent due to delays in the processing of that patent. There is nothing in the PTA statute to suggest that application of ODP to the PTA-extended patent term would be contrary to the congressional design…. * * * In Merck and Novartis, the holdings were premised on meaningful and substantive differences evincing a clear congressional intent to constitute PTE and PTA as different statutory frameworks. In particular, those cases set forth how § 154 clearly states that PTA “shall” be granted when certain requirements are met. 35 USC § 154(b)(1)(A), (b)(1)(B), and (b)(1)(C). But those requirements include limitations that are separate and distinct from those in the PTE framework, including the inability to extend a term past any date in a filed terminal disclaimer. Compare 35 USC § 154(b)(2)(B) with 35 USC § 156(c)(3), and (g)(6) (providing for statutory limitations on length of PTE and number of patents that can be extended). Slip Op. at 18. Since all of the challenged patents were expired, even after factoring in PTA, the Court further held that late-filed TD's were not available to save the Cellect patents from ODP: Terminal disclaimers, which may be filed to overcome an ODP rejection assuming that the first patent has not yet expired, are provided for in 35 USC § 253 and 37 CFR § 1.321. No terminal disclaimers were filed by Cellect, and the patents at issue have all expired, precluding any late filings of terminal disclaimers. Slip Op. at 16. The Cellect opinion has garnered substantial commentary from the patent community, increasing the possibility of further review, e.g.: - Dennis Crouch, Double Patenting and Patent Term Adjustment, Patently-O (Aug. 28, 2023)
- Eileen McDermott, CAFC Issues Precedential Ruling on Proper Analysis for Patent Term Adjustment in Double Patenting Cases, IPWatchdog (Aug. 28, 2023)
- Michael Shapiro, Court Broadens Judge-Created Rule Targeting ‘Double Patenting', Bloomberg Law (Aug. 28, 2023)
- Sherry Knowles, A Comment on In re Cellect: The Patent Bar Must Push for Eliminating ODP Altogether, Not Interpreting it More Favorably, IPWatchdog (Aug. 29, 2023)
- Wolf Greenfield, In re Cellect: What In-House Counsel Needs to be Doing Right Now, Wolf Greenfield IP Alerts (Aug. 29, 2023)
- Karina J. Moy et al., Federal Circuit: Patent Term Adjustment Results in Invalidity on Obviousness-Type Double Patenting Grounds, Akin Insights (Aug. 30, 2023)
- Kevin E. Noonan, In re Cellect (Fed. Cir. 2023), Patent Docs (Aug. 30, 2023)
- Timothy A. Cook et al., Patent Term Adjustments in Jeopardy After In re Cellect, WilmerHale News and Insights (Aug. 30, 2023)
- Goodwin Law, In re Cellect: Federal Circuit Opens New Path for Double Patenting Challenges, JD Supra (Aug. 31, 2023)
Applicants are advised to carefully consider the Cellect opinion and developing legal commentary on its impact when prosecuting interrelated patent families. The above overview is general in nature. Please see the Federal Circuit Opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
June 16, 2023: USPTO Requires Form PTO/SB/133 and Document Code "PTA.IDS" for PTA Safe Harbor Statements The USPTO has issued a Final Rule revising 37 CFR 1.704(d) to require that applicants use USPTO Form PTO/SB/133 and document code "PTA.IDS" when making a PTA Safe Harbor Statement for an Information Disclosure Statement (IDS) filing. Where an applicant files an IDS at certain points during prosecution, depending on the timing, the IDS may be considered applicant delay generating a PTA reduction. See 37 CFR 1.704(c)(6), (8), (9), (10), and (12). Common examples include filing an IDS after replying to an Office action or after a notice of allowance. Id. In appropriate circumstances, applicants can avoid this PTA reduction by filing the IDS accompanied by a 30-day Safe Harbor Statement under 37 CFR 1.704(d). For IDS filings on or after July 17, 2023, this Final Rule adds several requirements for a proper Safe Harbor Statement via new paragraph 37 CFR 1.704(d)(3): The statement under paragraph (d)(1) of this section must be submitted on the Office form (PTO/SB/133) provided for such a patent term adjustment statement using the appropriate document code (PTA.IDS). Otherwise, the paper or request for continued examination will be treated as not accompanied by a statement under paragraph (d)(1) of this section unless an application for patent term adjustment, in compliance with § 1.705(b), is filed, establishing that the paper or request for continued examination was accompanied by a statement in compliance with paragraph (d)(1) of this section. No changes to statements on this Office form may be made. The presentation to the Office (whether by signing, filing, submitting, or later advocating) of this form, whether by a practitioner or non-practitioner, constitutes a certification under § 11.18(b) of this chapter that the existing text and any certification statements on this form have not been altered. With this change, the USPTO states that using Form PTO/SB/133 and Document Code "PTA.IDS" will make the USPTO’s automated PTA algorithm more likely to properly account for Safe Harbor Statements and ensure that applicants include the required language of 37 CFR 1.704(d)(1), thus eliminating the need for unnecessary back-and-forth between the Office and applicant. While the Final Rule still allows applicants to submit their own 37 CFR 1.704(d)(1) Safe Harbor Statements without using Form PTO/SB/133 or the document code, an extra step is now required. After the patent issues, the patentee must file a timely Application for PTA in compliance with Rule 1.705(b), including the appropriate fee, to facilitate manual USPTO review of their statement. Accordingly, applicants are strongly advised to adopt the new 37 CFR 1.704(d)(3) requirements, if not doing so already, to avoid the need for this post-grant filing. The above overview is general in nature. Please see the Final Rule for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
September 19, 2022: Federal Circuit Upholds USPTO Denial of PTA for Appellate Review In SawStop Holding LLC v. Vidal, 2021-1537 (Fed. Cir. 2022), the US Court of Appeals for the Federal Circuit upheld the USPTO's denial of C-Delay for Board and District Court appeals of claim rejections in two applications. In each case, while one ground of rejection was removed on appeal, another new or existing ground remained, thus precluding a "[reversal of] an adverse determination of patentability" required for C-Delay. Furthermore, since the final issued claims were substantively different than the claims reviewed on appeal, neither patent "issued under a decision in the [appellate] review," another C-Delay requirement. C-Delay is a type of PTA under 35 USC 154(b)(1)(C) to compensate applicants for delays caused by derivation and interference proceedings, secrecy orders, and successful appeals during prosecution. At issue here is 35 USC 154(b)(1)(C)(iii) concerning appeals to the Board and Federal Courts: Subject to the limitations under paragraph (2), if the issue of an original patent is delayed due to ... (iii) appellate review by the [Board] or by a Federal court in a case in which the patent was [1] issued under a decision in the review [2] reversing an adverse determination of patentability, the term of the patent shall be extended 1 day for each day of the pendency of the ... review .... (emphasis and bracketed numbers added). For the first patent (’476), the Court held that, even though the Board overturned the examiner's obviousness rejection, the Board issued a new obviousness rejection, thus precluding "[reversal of] an adverse determination of patentability" as required for C-Delay: While there is no dispute that the Board cast aside the examiner’s basis for rejecting claim 11, the Board in the same review found claim 11 unpatentable, albeit for a different reason. The adverse determination of unpatentability remained before and after the appeal to the Board. The appeal thus resulted in no substantive change in the patentability of claim 11. Such a substantive change is required by the language of the statute itself: the reversal of a “determination of patentability” requires a determination that the claim in question is substantively allowable, not just free of a particular rejection.... We reject Sawstop’s attempt to rewrite the statutory text to mandate PTA over reversal of a mere “rejection” or “basis for unpatentability.” Here, the appeal of the determination of patentability of claim 11 was not “successful” as it was not “reversed.” The PTO faithfully applied the statutory text and did not improperly add a new requirement for eligibility for (C) delay. (citations omitted). The Court also held that the '476 patent did not “issue[] under a decision in the review” because of subsequent substantive claim amendments: Under Sawstop’s reading, the (C) delay provision could apply when an adverse determination of patentability is overcome on appeal, regardless of substantive amendments made after the appeal to secure allowance. This interpretation effectively reads out the phrase “in which the patent was issued under a decision in the review,” and thus cannot be sustained.... The plain language of “issued under a decision in the review” means that at least one claim must “issue[] under” the mandate of the appellate decision. At a minimum, this means that at least one claim that “issued” must have been analyzed by the Board or District Court that issued the “decision in the review.” The statutory requirement is not met if the claim that ultimately issues differs substantively from the claim under review. (citation omitted). For the second patent (’796), the Court held the presence of an outstanding provisional double patenting rejection likewise precluded C-Delay for a District Court appeal: The problem with Sawstop’s position is that the PTO’s “adverse determination of patentability” of claim 1 of the ’796 patent was based on two grounds: double patenting and anticipation. Sawstop only appealed anticipation without addressing the provisional double patenting rejection. As a result, the District Court for the District of Columbia understandably did not address the double patenting rejection. Sawstop’s success in reversing the anticipation rejection left the provisional double patenting rejection in place. Claim 1 of the ’796 patent was thus unpatentable both before the appeal (because of anticipation and double patenting) and after the appeal (because of double patenting). Like the ’476 patent, the appellate decision did not reverse an adverse determination of patentability. (citation omitted). The Court further held that the ’796 patent was also ineligible for C-Delay because it did not "issue[] under a decision in the review," since the appealed claim was cancelled and not part of the issued patent. To maximize PTA, applicants are advised to carefully consider the Sawstop requirements for C-Delay when undertaking a Board or Federal Court appeal, and before making any claim cancellations or amendments after a successful appeal (including certain remands per 37 CFR 1.702(e)). The above overview is general in nature. Please see the Federal Circuit Opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
January 07, 2022: USPTO Weather-Related Closing The USPTO was closed on Friday, January 7, 2022. See USPTO Status page. Per 35 USC 21, where a 3-month 1.704(b) applicant response deadline falls on this date, the effective 3-month deadline for determining any PTA reduction is extended until Monday, January 10, 2022. See ArQule v. Kappos, 793 F.Supp.2d 214 (D.D.C. 2011); MPEP § 2732. We have updated our calculator to automatically consider this closing in analyzing PTA rules when the Apply ArQule v. Kappos option (on by default) is selected on the Apply Term Rules tab. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
January 03, 2022: USPTO Weather-Related Closing The USPTO was closed on Monday, January 3, 2022. See USPTO Status page. Per 35 USC 21, where a 3-month 1.704(b) applicant response deadline falls on this date, the effective 3-month deadline for determining any PTA reduction is extended until Tuesday, January 4, 2022. See ArQule v. Kappos, 793 F.Supp.2d 214 (D.D.C. 2011); MPEP § 2732. We have updated our calculator to automatically consider this closing in analyzing PTA rules when the Apply ArQule v. Kappos option (on by default) is selected on the Apply Term Rules tab. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
February 18, 2021: USPTO Weather-Related Closing The USPTO was closed on Thursday, February 18, 2021. See USPTO Status and OPM Status pages. Per 35 USC 21, where a 3-month 1.704(b) applicant response deadline falls on this date, the effective 3-month deadline for determining any PTA reduction is extended until Friday, February 19, 2021. See ArQule v. Kappos, 793 F.Supp.2d 214 (D.D.C. 2011); MPEP § 2732. We have updated our calculator to automatically consider this closing in analyzing PTA rules when the Apply ArQule v. Kappos option (on by default) is selected on the Apply Term Rules tab. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
February 11, 2021: Federal Circuit Upholds USPTO C-Delay Calculation In Chudik v. Hirshfeld, 2020-1833 (Fed. Cir. 2021), the US Court of Appeals for the Federal Circuit upheld the USPTO's position that PTA for appellate review is not available unless there is an actual decision by the Patent Trial and Appeal Board or a court reversing an an adverse determination of patentability. Where an examiner reopens prosecution in response to a notice of appeal without Board review, PTA for C-Delay is not available. C-Delay is a type of PTA under 35 USC 154(b)(1)(C) to compensate applicants for delays caused by derivation and interference proceedings, secrecy orders, and appeals during prosecution. At issue here is 35 USC 154(b)(1)(C)(iii) concerning appeals to the Board and Federal courts: Subject to the limitations under paragraph (2), if the issue of an original patent is delayed due to ... (iii) appellate review by the [Board] or by a Federal court in a case in which the patent was issued under a decision in the review reversing an adverse determination of patentability, the term of the patent shall be extended 1 day for each day of the pendency of the ... review .... The USPTO has prescribed two related regulations. Rule 1.702(e) pertains to “[d]elays caused by successful appellate review” providing: [T]he term of an original patent shall be adjusted if the issuance of the patent was delayed due to review by the [Board] under 35 USC 134 or by a Federal court under 35 USC 141 or 145, if the patent was issued under a decision in the review reversing an adverse determination of patentability. Rule 1.703(e), the other regulation, explains how to calculate C-Delay for appeals: The period of adjustment under § 1.702(e) is the sum of the number of days, if any, in the period beginning on the date on which jurisdiction passes to the [Board] under § 41.35(a) of this chapter and ending on the date of a final decision in favor of the applicant by the [Board] .... Prosecution of the subject Chudik patent lasted over eleven and a half years. It included one Request for Continued Examination, and four notices of appeal and appeal briefs filed by the applicant. In response to each of the first three appeals, rather than letting the case proceed to the Board, the examiner reopened prosecution rejecting the claims on different grounds. Finally, after the fourth appeal, the examiner issued yet another new rejection, but this one eventually led to allowance. Despite this lengthy piecemeal examination and multiple notices of appeal, the Court held that 35 USC 154(b)(1)(C)(iii) cannot provide PTA to compensate for term loss since there was no Board decision: The statute’s words, in their most natural meaning when applied to an examiner’s unpatentability ruling, require that the patent issue under a Board decision that reversed the examiner’s unpatentability ruling or under a court decision that reversed a Board unpatentability ruling in the matter. Dr. Chudik’s interpretation—that the provision also covers an examiner’s reopening that withdraws a rejection—is, if not linguistically impossible, strained.... [I]n an appellate review, the normal meaning of “reverse” is an action by the reviewer with respect to the decision being reviewed. This ordinary meaning does not include the examiner’s repeated reopening in this case to withdraw her own decision. Slip Op. at 11 (citation omitted). The Court did not address the USPTO's other ground for its denial, that there is no PTA since Board jurisdiction over the appeal never attached as required by 37 CFR 1.703(e). The Court also did not consider what type of Board decisions might or might not be considered a reversal. The Court noted that B-Delay, a different type of PTA for prosecution time exceeding three years, could have potentially compensated for some of the substantial term loss if the applicant had not filed an RCE before its appeals: [N]o such B-delay increase occurred because Dr. Chudik, rather than appealing to the Board after his 2010 final rejection, sought continued examination, triggering a statutory exclusion from the time counted for a B-delay adjustment. See 35 USC 154(b)(1)(B)(i) (excluding “any time consumed by continued examination of the application requested by the applicant under [35 USC 132(b)]”). The unavailability of B-delay for nearly two years (655 days) of delay in the PTO illustrates what applicants should understand when deciding whether to request a continued examination rather than take an immediate appeal. The potential benefit of immediate re-engagement with the examiner through such continued examination comes with a potential cost. Slip Op. at 15 (citation omitted). The above overview is general in nature. Please see the Federal Circuit Opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
June 16, 2020: USPTO Revises PTA Rules in view of Supernus [Updated] The USPTO has issued a Final Rule revising PTA reductions for six types of applicant delay in view of the Federal Circuit decision in Supernus v. Iancu, 913 F.3d 1351 (Fed. Cir. 2019). Supernus overturned a PTA reduction under 37 CFR 1.704(c)(8), based on the applicant's filing of an IDS after an RCE, holding that any PTA reduction must be equal to the period of time during which the applicant failed to engage in reasonable efforts to conclude prosecution of the application per 35 USC 154(b)(2)(C)(i). The Court held that PTA cannot be reduced for periods in which there is no identifiable effort in which an applicant could have engaged to conclude prosecution. See posts below for further details of the Supernus holding. The American Inventor’s Protection Act (AIPA) grants term adjustment to compensate patentees for certain USPTO prosecution delays set forth in 35 USC 154(b)(1). From this positive adjustment, 35 USC 154(b)(2)(C)(i) mandates that applicant delay be subtracted to compute the final PTA: The period of adjustment of the term of a patent ... shall be reduced by a period equal to the period of time during which the applicant failed to engage in reasonable efforts to conclude prosecution of the application. By this revision, the USPTO seeks to ensure such PTA reductions correspond to actual periods of applicant failure-to-engage conduct, rather than periods which may correspond to or include the consequences of such conduct to the USPTO. For example, under the revised rule, an abandonment reduction will now end when an applicant files a grantable petition to revive (its last identifiable effort), rather than continuing the reduction up to 4 additional months until the USPTO grants the petition. Cf. 37 CFR 1.704(c)(3) (2015) with 37 CFR 1.704(c)(3) (2020). Specifically the Final Rule revises six types of failure-to-engage conduct (applicant delay) set forth in 37 CFR 1.704(c) as follows: | Revision | Notes |
|---|
| (2) Deferral of issuance of a patent under § 1.314, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the date a request for deferral of issuance of a patent under §1.314 was filed and ending on the earlier of the date a request to terminate the deferral was filed or the date the patent was issued; | This rule seldom applies. Only affects applications where issuance was deferred. Revision may reduce applicant delay. | (3) Abandonment of the application or late payment of the issue fee, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the date of abandonment or the date day after the date the issue fee was due and ending on the earlier of: (i) The date of mailing of the decision reviving the application or accepting late payment of the issue fee; or (ii) The date that is four months after the date the grantable petition to revive the application or accept late payment of the issue fee was filed; | Only affects applications which were abandoned (including late payment of issue fee) and revived. Revision will reduce applicant delay up to four months (for each abandonment). | (4) Failure to file a petition to withdraw the holding of abandonment or to revive an application within two months from the mailing date date of mailing of a notice of abandonment, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the day after the date two months from the mailing date date of mailing of a notice of abandonment and ending on the date a petition to withdraw the holding of abandonment or to revive the application was filed; | Minor wording change for consistency unlikely to affect PTA. | (6) Submission of a preliminary amendment or other preliminary paper less than one month before the mailing of an Office action under 35 USC 132 or notice of allowance under 35 USC 151 that requires the mailing of a supplemental Office action or notice of allowance, in which case the period of adjustment set forth in § 1.703 shall be reduced by lesser of: (i) The number of days, if any, beginning on the day after the mailing date of the original Office action or notice of allowance and ending on the date of mailing of the supplemental Office action or notice of allowance; or (ii) Four months; the number of days, if any, beginning on the day after the date that is eight months from either the date on which the application was filed under 35 USC 111(a) or the date of commencement of the national stage under 35 USC 371(b) or (f) in an international application and ending on the date the preliminary amendment or other preliminary paper was filed; | This rule seldom applies. Only affects applications where a preliminary amendment or certain preliminary applicant papers (1) were filed less than one month before the first Office action/allowance; and (2) require the mailing of a supplemental action/allowance. Revision may increase or decrease applicant delay depending on circumstances. Increase could possibly be substantial since 4 month cap is removed. | (9) Submission of an amendment or other paper after a decision by the Patent Trial and Appeal Board, other than a decision designated as containing a new ground of rejection under § 41.50 (b) of this title or statement under § 41.50(c) of this title, or a decision by a Federal court, less than one month before the mailing of an Office action under 35 USC 132 or a notice of allowance under 35 USC 151 that requires the mailing of a supplemental Office action or supplemental notice of allowance, in which case the period of adjustment set forth in § 1.703 shall be reduced by the lesser of: (i) The number of days, if any, beginning on the day after the mailing date of the original Office action or notice of allowance and ending on the mailing date of the supplemental Office action or notice of allowance; or (ii) Four months; the number of days, if any, beginning on the day after the date of the decision by the Patent Trial and Appeal Board or by a Federal court and ending on date the amendment or other paper was filed; | This rule seldom applies. Only affects applications where an amendment or certain applicant papers (1) were filed after a PTAB or Court decision (not including some PTAB decisions containing a new ground of rejection); (2) were also filed less than one month before an Office action/allowance; and (3) require the mailing of a supplemental action/allowance. Revision may increase or decrease applicant delay depending on circumstances. Increase could possibly be substantial since 4 month cap is removed. | (10) Submission of an amendment under § 1.312 or other paper, other than an amendment under § 1.312 or other paper expressly requested by the Office or a request for continued examination in compliance with § 1.114, after a notice of allowance has been given or mailed, in which case the period of adjustment set forth in § 1.703 shall be reduced by the lesser of: (i) The number of days, if any, beginning on the date the amendment under § 1.312 or other paper was filed and ending on the mailing date of the Office action or notice in response to the amendment under § 1.312 or such other paper; or (ii) Four months; the number of days, if any, beginning on the day after the date of mailing of the notice of allowance under 35 USC 151 and ending on the date the amendment under § 1.312 or other paper was filed; | This rule is common and the revision will affect many applications. Applies where a 312 amendment or many other applicant papers (e.g, IDS without 1.704(d) statement) are filed after allowance. Revision may increase or decrease applicant delay depending on circumstances, but will often increase delay which now begins the day after allowance. Increase could possibly be substantial since 4 month cap is removed. Filing papers expressly requested by the USPTO, however, will no longer be considered applicant delay, which may increase PTA. | Discussion in the Final Rule provides guidance on the meaning of "expressly requested by the Office" for purposes of exempting post-allowance applicant papers from the Rule 1.704(c)(10) PTA reduction: An amendment under § 1.312 or other paper going beyond what was requested by the USPTO (i.e., including material not expressly requested by the USPTO in addition to what was requested by the USPTO) would not be considered “an amendment under § 1.312 or other paper expressly requested by the Office” under § 1.704(c)(10). In addition, the phrase “expressly requested by the Office” requires a specific request in an Office action or notice, or in an Examiner’s Interview Summary (PTOL-413), for the amendment under § 1.312 or other paper. For example, generic language in an Office action or notice, such as a statement in a notice of allowability containing an examiner’s amendment indicating that if the changes and/or additions are unacceptable to applicant, an amendment may be filed as provided by § 1.312 (section 1302.04 of the [MPEP]), is not a basis for considering an amendment under § 1.312 to be “expressly requested by the Office” within the meaning of § 1.704(c)(10) as adopted in this final rule. Similarly, the provisions of §§ 1.56, 1.97, and 1.98 are not a basis for considering an [IDS] including information that has come to the attention of the applicant after a notice of allowance has been given or mailed to be a paper “expressly requested by the Office” within the meaning of § 1.704(c)(10). The Final Rule is effective on July 16, 2020 and its changes apply to PTA-eligible patents issuing from applications in which a notice of allowance was mailed on or after July 16, 2020. Additionally, the USPTO will apply the changes to earlier patents if requested by the patentee in a compliant request for reconsideration of PTA under 37 CFR 1.705(b). Since the 37 CFR 1.705(b) request deadline is within 2 months from the patent grant date, extendable by an additional 5 months pursuant to Rule 1.136(a), patentees may request retroactive application for patents issuing up to about 7 months prior to the request date (a few extra days might be available if the request was filed after a weekend or holiday). This could provide a term benefit for some important patents (including pending applications allowed prior to July 16, 2020) where, e.g., PTA was reduced for abandonment or post-allowance papers expressly requested by the USPTO. Patentees of course will need to consider the overall PTA effect of the revision. The USPTO is in the process of modifying its patent term adjustment calculation program to implement the changes in the Final Rule. During this process, patentees are advised to carefully check USPTO-calculated PTA in light of the rule change and seek correction in appropriate cases. We will be adding options to our calculator to automatically perform analysis in accordance with the Final Rule prior to its effective date. The above overview is general in nature. Please see the Final Rule for complete details. If you have questions, please contact us by email to Support@PatentTerm.com.
May 17, 2020: Federal Circuit Upholds USPTO A-Delay Calculation In Idorsia Pharm. v. Iancu, 2019-2346 (Fed. Cir. 2020), a nonprecedential opinion, the US Court of Appeals for the Federal Circuit upheld the USPTO's PTA calculation of A-Delay for US Patent 8,518,912. The Court held that an erroneous restriction requirement, even though twice superseded and replaced, nonetheless constitutes a first Section 132 action for purposes of the USPTO's 14-month first action deadline under 35 USC 154(b)(1)(A)(i). A-Delay is a type of PTA under 35 USC 154(b)(1)(A) accruing if the USPTO fails to act by certain examination deadlines. The deadline at issue in Idorsia requires the USPTO to provide a Section 132 notification (e.g., a restriction requirement or Office action), or a notice of allowance, within 14 months of the filing or national stage commencement date. For each day the USPTO misses its deadline, one day of PTA accrues. See 35 USC 154(b)(1)(A)(i). In the prosecution of the patent on appeal, the USPTO issued a series of three restriction requirements: | | Details | Response | | First Restriction Requirement issued March 14, 2012 | Identifies six invention groups for all pending claims that were "independent and distinct from each other because they [we]re directed to structurally dissimilar compounds that lack a common core" based on the possible variations for the group. States "[r]estriction [wa]s required under 35 USC 121." | Applicant notified examiner by telephone that the invention groups omitted certain subject matter from the scope of the claims. Examiner agreed and indicated he would issue a new restriction. | | Second Restriction Requirement issued April 18, 2012 | Supersedes and replaces first restriction requirement and divides all pending claims into eight distinct invention groups. | Applicant notified examiner by telephone that the invention groups omitted claimed subject matter. Examiner agreed and indicated he would issue a third restriction requirement. Applicant did not elect any groups. | | Third Restriction Requirement issued June 21, 2012 | Divides all pending claims into three distinct invention groups. | Applicant filed a typical response to the third restriction requirement, electing one of the three invention groups, and traversing the restriction. | In its challenge to the USPTO-calculated PTA, Idorsia argues that the third restriction requirement, not the earlier erroneous ones, ends the USPTO 14-month deadline, thus extending USPTO A-Delay and requiring a greater term adjustment. The Court rejected this contention, relying extensively on the 2016 Pfizer decision (see discussion below), holding inter alia: In Pfizer, we held that § 132 "merely requires that an applicant at least be informed of the broad statutory basis for [the rejection of] his claims, so that he may determine what the issues are on which he can or should produce evidence." We explained that § 132 also requires that the examiner's rejection be "sufficiently informative to allow [the applicant] to counter the grounds for rejection." As to this second requirement for notice, we reaffirmed our precedent holding that § 132 "is violated when a rejection is so uninformative that it prevents the applicant from recognizing and seeking to counter the grounds for rejection." * * * In this case, Idorsia does not dispute that the examiner's first restriction requirement provided notice of the statutory basis for the examiner's rejection, namely, that restriction was required under 35 USC § 121. Rather, the parties' dispute focuses on whether the first restriction requirement was "sufficiently informative to allow [Applicant] to counter the grounds for rejection. ..." [Applicant] was able to respond to the first restriction requirement and successfully oppose the examiner's description of the multiple invention groups, which demonstrates that [Applicant] was able to understand the examiner's proposed invention groups and prepare responsive arguments. * * * ... [Applicant's] and the examiner's "exchanges concerning the challenged restriction requirement were part of the typical 'back and forth' process of patent prosecution." Although this process "often involves changes in both the applicant's and examiner's positions, an examiner's reissuance of an office action in response to an applicant's suggestion does not automatically mean that an application has been 'delayed' for purposes of patent term adjustment." Slip Op. at 6 (citations and internal quotations omitted). Note that, in contrast to the effect of such USPTO conduct, where an applicant submits a reply having an omission, or supplements its reply (unless expressly requested by the Examiner), such actions will typically generate a PTA reduction. See 37 CFR 1.704(c)(7),(8). The above overview is general in nature. Please see the Federal Circuit Opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
October 07, 2019: USPTO Proposes PTA Rule Changes in view of Supernus The USPTO has issued a Notice proposing substantial PTA rule changes in view of the Federal Circuit decision in Supernus v. Iancu, 913 F.3d 1351 (Fed. Cir. 2019). Supernus overturned a PTA reduction under 37 CFR 1.704(c)(8), based on the applicant's filing of an IDS after an RCE, holding that any PTA reduction must be equal to the period of time during which the applicant failed to engage in reasonable efforts to conclude prosecution of the application per 35 USC 154(b)(2)(C)(i). The Court held that PTA cannot be reduced for periods in which there is no identifiable effort in which an applicant could have engaged to conclude prosecution. See below, January 23, 2019, for further details of the Supernus holding. By this revision, the USPTO seeks to ensure PTA reductions correspond to periods of applicant failure-to-engage conduct, rather than periods which may correspond to or include the consequences of such conduct in the USPTO. For example, an abandonment reduction would end when an applicant files a grantable petition to revive, rather than when the USPTO grants the petition. See 37 CFR 1.704(c)(3) (note USPTO action time is capped at 4 months). For several subsections, however, the start of applicant delay is redefined which may increase the PTA reduction. See, e.g., 37 CFR 1.704(c)(10). The Notice proposes revisions to five paragraphs of 37 CFR 1.704(c): Circumstances that constitute a failure of the applicant to engage in reasonable efforts to conclude processing or examination of an application also include ... (2) Deferral of issuance of a patent under §1.314, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the date a request for deferral of issuance of a patent under §1.314 was filed and ending on the earlier of the date a request to terminate the deferral was filed or the date the patent was issued; (3) Abandonment of the application or late payment of the issue fee, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the date of abandonment or the date after the date the issue fee was due and ending on the earlier of: (i) The date of mailing of the decision reviving the application or accepting late payment of the issue fee; or (ii) The date that is four months after the date the grantable petition to revive the application or accept late payment of the issue fee was filed; (6) Submission of a preliminary amendment or other preliminary paper less than one month before the mailing of an Office action under 35 USC 132 or notice of allowance under 35 USC 151 that requires the mailing of a supplemental Office action or notice of allowance, in which case the period of adjustment set forth in § 1.703 shall be reduced by lesser of: (i) The number of days, if any, beginning on the day after the mailing date of the original Office action or notice of allowance and ending on the date of mailing of the supplemental Office action or notice of allowance; or (ii) Four months; the number of days, if any, beginning on the day after the date that is eight months from either the date on which the application was filed under 35 USC 111(a) or the date of commencement of the national stage under 35 USC 371(b) or (f) in an international application and ending on the date the preliminary amendment or other preliminary paper was filed; (9) Submission of an amendment or other paper after a decision by the Patent Trial and Appeal Board, other than a decision designated as containing a new ground of rejection under § 41.50 (b) of this title or statement under § 41.50(c) of this title, or a decision by a Federal court, less than one month before the mailing of an Office action under 35 USC 132 or notice of allowance under 35 USC 151 that requires the mailing of a supplemental Office action or supplemental notice of allowance, in which case the period of adjustment set forth in § 1.703 shall be reduced by the lesser of: (i) The number of days, if any, beginning on the day after the mailing date of the original Office action or notice of allowance and ending on the mailing date of the supplemental Office action or notice of allowance; or (ii) Four months; the number of days, if any, beginning on the day after the date of the decision by the Patent Trial and Appeal Board or by a Federal court and ending on date the amendment or other paper was filed; (10) Submission of an amendment under § 1.312 or other paper, other than a request for continued examination in compliance with § 1.114, after a notice of allowance has been given or mailed, in which case the period of adjustment set forth in § 1.703 shall be reduced by the lesser of: (i) The number of days, if any, beginning on the date the amendment under § 1.312 or other paper was filed and ending on the mailing date of the Office action or notice in response to the amendment under § 1.312 or such other paper; or (ii) Four months; the number of days, if any, beginning on the day after the mailing date of the notice of allowance under 35 USC 151 and ending on the date the amendment under § 1.312 or other paper was filed; Based on our observations, PTA reductions under Paragraphs (2), (6), and (9) are fairly rare, and PTA reductions under Paragraph (3) are not too common. PTA reductions under Paragraph (10) are common, however, and the revision will impact many applications. For example, Rule 1.704(c)(10) is invoked whenever, after allowance, an applicant files Rule 312 amendments, priority claims or corrections including a new or supplement ADS correcting benefit information, requests for a corrected filing receipt, certified copies of priority documents, drawings, letters relating to biologic deposits, requests to change or correct inventorship, and IDS's without a 1.704(d) statement. See MPEP 2732. The likely overall effect will be an increase in PTA reduction since the current Rule 1.704(c)(10) reduction is often small (e.g., the period from a request for a corrected filing receipt until a corrected filing receipt is issued) and is capped at 4 months, but the result will vary in individual cases. The Notice does not specifically address retroactivity, and consideration of the effect on pending applications and issued patents will be needed. We will be submitting comments to the USPTO and will provide further information on this page. Comments are due on or before December 3, 2019. The above overview is general in nature. Please see the Notice for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
September 24, 2019: Federal Circuit Upholds USPTO PTA Reduction for Applicant Delay In Intra-Cellular Therapies, Inc. v. Iancu, 2018-1849 (Fed. Cir. 2019), the US Court of Appeals for the Federal Circuit upheld the USPTO's PTA reduction in US Patent 8,648,077 for applicant's late response to a final Office action. 35 USC 154(b)(2)(C)(i) mandates that applicant delay be subtracted from USPTO delay to compute PTA. One such applicant delay is taking more than 3 months to respond to a USPTO notice. 35 USC 154(b)(2)(C) provides: The period of adjustment of the term of a patent ... shall be reduced by a period equal to the period of time during which the applicant failed to engage in reasonable efforts to conclude prosecution of the application.... [A]n applicant shall be deemed to have failed to engage in reasonable efforts to conclude processing or examination of an application for the cumulative total of any periods of time in excess of 3 months that are taken to respond to a notice from the Office making any rejection, objection, argument, or other request, measuring such 3-month period from the date the notice was given or mailed to the applicant. 37 CFR 1.704(b), promulgated by the USPTO under its authority to "prescribe regulations establishing the circumstances that constitute a failure of an applicant to engage in reasonable efforts," see 35 USC 154(b)(2)(C)(iii), also makes this requirement: [A]n applicant shall be deemed to have failed to engage in reasonable efforts to conclude processing or examination of an application for the cumulative total of any periods of time in excess of three months that are taken to reply to any notice or action by the Office making any rejection, objection, argument, or other request, measuring such three-month period from the date the notice or action was mailed or given to the applicant, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the day after the date that is three months after the date of mailing or transmission of the Office communication notifying the applicant of the rejection, objection, argument, or other request and ending on the date the reply was filed.... The Intra-Cellular dispute involves the following prosecution events: | April 17, 2013 | Final Office Action | Rejects or objects to all outstanding claims. Repeats prior 35 USC 103 rejections; finds applicant's amendments do not overcome other rejections and objections; adds new objections to newly introduced informalities. | | July 17, 2013 | Amendment and Response after Final | Applicant continues to dispute the 103 rejections using the same arguments; amends claims to address other objections and rejections; adds new claim. | | July 26, 2013 | Advisory Action | Indicates after-final submission overcame some 112 rejections and formality objections, but fails to overcome 103 rejections for the prior reasons of record. Suggests amending or cancelling certain claims to overcome 103 rejections and 112 objections to place application in better condition for allowance. Enters amendments for purposes of appeal. | | August 7, 2013 | Amendment and Response to Advisory Action | Adopts all of the examiner's suggestions for overcoming outstanding rejections and objections. | | August 20, 2013 | Notice of Allowance | Allows remaining claims. | Regarding applicant's 3-month deadline to reply to the final Office action, the USPTO assessed 21 days of applicant delay, concluding that applicant's first submission on July 17, 2013 was not a proper reply under 37 CFR 1.113(c), and thus applicant failed to meet its deadline until its second submission on August 7, 2013. In contrast, Intra-Cellular argued its first submission met the deadline, since it was a bona fide attempt to advance prosecution and addressed all outstanding objection and rejections, thus constituting "reasonable efforts to conclude prosecution." After finding the plain language of the PTA Statute did not resolve the issue, the Court upheld the USPTO's reliance on 37 CFR 1.113(c) in determining whether an after-final submission constitutes a reply for purposes of 37 CFR 1.704(b), as a permissible construction of the Statute: While § 1.704(b) does not explicitly define "reply," that does not mean that any type of submission by the applicant, no matter how flimsy or superficial, necessarily qualifies as a "reply" for purposes of stopping accrual of applicant delay. Thus, some standard of compliance must be used, and the Patent Office already had longstanding regulatory standards in place for a complete and proper reply—one for replies to non-final Office actions (§ 1.111) and one for replies to final Office actions (§ 1.113). Given that the submission at issue was filed in response to a final Office action, the Patent Office appropriately turned to preexisting regulatory requirements set forth in § 1.113 for responding to a final Office action, unchallenged here, in interpreting whether Intra-Cellular's after-final submission qualified as a "reply" under § 1.704(b). * * * Section 1.113(a) sets forth limited ways for an applicant to properly respond to a final Office action. It provides that an applicant's "reply is limited to appeal in the case of rejection of any claim . . . or to amendment as specified in § 1.114 or § 1.116." It also states that a "[r]eply to a final rejection or action must comply with § 1.114 or paragraph (c) of this section." Thus, § 1.113(a) at minimum sets forth two ways to file a proper reply to a final Office action-by either complying with § 1.114 (filing an RCE) or § 1.113(c) (cancellation or appeal of rejected claims). While § 1.113(a) also indicates that a § 1.116 amendment can be filed, § 1.116 itself indicates that a § 1.116 amendment filed by an applicant, without more, does not necessarily relieve the applicant's responsibility to timely reply to a final Office action. As § 1.116(c) provides, the "admission of, or refusal to admit, any amendment after a final rejection, a final action, an action closing prosecution, or any related proceedings, will not operate to relieve the application ... from its condition as subject to appeal or save the application from abandonment." Thus, the regulatory framework makes clear that for purposes of responding to a final Office action rejecting at least some of the claims, a proper "reply" must either comply with § 1.113(c) or § 1.114. The Patent Office properly read the term "reply" in § 1.704(b) in harmony with those regulatory requirements for determining whether an applicant's response to a final Office action cuts off accrual of applicant delay. Slip Op. at 16 (emphasis added, citations omitted). Accordingly, since Intra-Cellular's first submission did not comply with 1.113(c) (cancellation or appeal of rejected claims) or 1.114 (filing an RCE), the Court agreed it did not constitute a "reply" ending applicant delay: In that first after-final submission, Intra-Cellular continued to dispute the § 103 rejection with the same arguments the examiner had previously found unpersuasive in overcoming the same § 103 rejection in the non-final Office action. Because prosecution was closed and the examiner at that point was under no obligation to reconsider arguments that had already been rejected in the final Office action, such applicant conduct does not amount to "reasonable efforts to conclude" prosecution under § 1.704(b). Until a compliant reply was filed, Intra-Cellular began accruing "applicant delay" once the three-month deadline passed for responding to the final Office action. * * * [U]nder Intra-Cellular's interpretation, an applicant would be allowed to continue to liberally argue and make amendments without accruing applicant delay as long as it addressed all outstanding issues in the final Office action. But treating this type of submission as a proper "reply" would give the applicant the benefits of an RCE (which re-opens prosecution) without the concomitant PTA reduction that comes with an RCE. This clearly contravenes the structure of the existing PTA statute, which prevents extension of PTA through B Delay accrual for time consumed by an RCE. Slip Op. at 17 (emphasis added, citations omitted). The Court did not address the effect, if any, of the various after-final pilot programs (see, e.g., 78 FR 29117) on applicant delay since such programs were not used in the subject prosecution or argued by Intra-Cellular. Additionally, in footnote 2, the Court questioned the USPTO's assertion that its "reply having an omission" rule, 37 CFR 1.704(c)(7), provides alternate support for the PTA reduction: The Patent Office's alternative reliance on § 1.704(c)(7) to justify accrual of applicant delay resulting from an improper reply to a final Office action is questionable. Section 1.704(c)(7) appears to apply only to replies to non-final Office actions. According to § 1.704(c)(7), a "[s]ubmission of a reply having an omission (§ 1.135(c))" constitutes a "circumstance[] that constitute[s] a failure of the applicant to engage in reasonable efforts to conclude processing or examination of an application" under § 1.704(c). But § 1.135(c) only addresses omissions made in a reply to a "non-final Office action." § 1.135(c) (referring to a situation where a "reply by the applicant is a bona fide attempt to advance the application to final action, and is substantially a complete reply to the non-final Office action, but consideration of some matter or compliance with some requirement has been inadvertently omitted" (emphasis added)). This seemingly contradicts the USPTO position that Rule 1.704(c)(7) "is not limited to Office actions under 37 CFR 1.135(c) but applies also when the Office issues any action or notice indicating that a reply has an omission which must be corrected," e.g., a petition dismissed as incomplete or a non-compliant reply to a notice to comply with sequence listings. See MPEP 2732; 65 FR 56366 at 56372 (Sept. 18, 2000). The above overview is general in nature. Please see the Federal Circuit Opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
September 17, 2019: Federal Circuit Upholds USPTO B-Delay Calculation In Mayo Foundation v. Iancu, 2018-2031 (Fed. Cir. 2019), a split panel of the US Court of Appeals for the Federal Circuit upheld the USPTO's PTA calculation of B-Delay for US Patent 8,981,063. The crux of the ruling is determining the amount of "time consumed by continued examination" where a patent's prosecution includes an interference declared after an RCE has been filed. B-Delay is a type of PTA under 35 USC 154(b)(1)(B) accruing if the USPTO fails to issue a patent within three years, not including specified exclusion periods. During such exclusions, the 3-year deadline is tolled and B-Delay does not accrue. The most common exclusion is for continued examination, see 35 USC 154(b)(1)(B)(i): Guarantee of no more than 3-year application pendency-- Subject to the limitations under paragraph (2), if the issue of an original patent is delayed due to the failure of the United States Patent and Trademark Office to issue a patent within 3 years after the actual filing date of the application ..., not including-- (i) any time consumed by continued examination of the application requested by the applicant under section 132(b) ..., the term of the patent shall be extended 1 day for each day after the end of that 3-year period until the patent is issued. In a previous decision, Novartis v. Lee, 740 F.3d 593 (Fed. Cir. 2014), the Federal Circuit overturned a prior USPTO regulation defining the period of continued examination, 37 CFR 1.703(b)(1) (2013), holding that "time consumed by continued examination" ends when an application is allowed: [T]he PTO contends that any time up until the patent issues, even after allowance, should be excluded from the adjustment awarded to the patentee. We reject the PTO’s view that the time after allowance, until issuance, is “time consumed by continued examination” and so is excluded from adjustments given to the patentee. * * * The language of "examination" used in 154(b)(1)(B) ... presumptively ends at allowance, when prosecution is closed and there is no further examination on the merits in the absence of a special reopening.... The common-sense understanding of "time consumed by continued examination," 35 USC 154(b)(1)(B)(i), is time up to allowance, but not later, unless examination on the merits resumes. 740 F.3d at 602. In response to Novartis, the USPTO revised 37 CFR 1.703(b)(1) to end "time consumed by continued examination" upon the mailing of a notice of allowance. See Final Rule, 80 FR 1346 (Jan. 9, 2015). In this appeal, the primary issue is whether an interference declaration, indicating some claims are otherwise in condition for allowance, effectively ends "continued examination" like a notice of allowance does. This would allow time consumed by post-interference examination (period 3 below), sua sponte reopened by the USPTO, to accrue B-Delay: The Mayo Court rejected this contention, upholding revised 37 CFR 1.703(b)(1): We agree with the PTO. While the PTO's regulations do indicate that at least one claim in an application should be in condition for allowance before an interference is declared, see 37 CFR 41.102, the regulations also explicitly contemplate that the Board may recommend further action by the examiner, including issuing a rejection. Id. 41.127(c); see also MPEP 2308 (instructing examiners to update their prior art search after remand and advising that "[an interference] judgment does not prevent the examiner from making a rejection in further examination in the same application or a different application"). Thus, the PTO's regulations as a whole do not indicate that a declaration of an interference is tantamount to a Notice of Allowance. * * * ...[Continued examination] is not just an opportunity to seek rehearing of a final rejection, and, as in regular examination, the PTO&'s regulations allow the examiner to issue a new ground of rejection, as was the case here. Mayo requested continued examination, and that is what it received, both before and after the interference proceeding. * * * We are also persuaded by the PTO's point that Mayo's rule could require an unduly burdensome, fact-intensive inquiry into when the PTO actually conceded the allowability of the claims. This is a case in point. Mayo claims that, after it filed its RCE, the examiner "indicated" on a phone call that its claims were in condition for allowance but for the interference, or, in Mayo's words, were "deemed allowable." The PTO understandably wishes to avoid such disputes over what "indicated" means and when claims are "deemed allowable." Slip Op. at 10-12 (some citations omitted). Judge Newman dissented, indicating that the post-interference examination delay is properly attributable to the USPTO and should generate PTA under some section of the statute: Here the post-interference examination was PTO activity, part of the examination procedure before issuance of the notice of allowance. It plainly is within the purpose of the term adjustment statute. Indeed, were this post-interference activity deemed part of interference delay, as my colleagues' ruling requires, this examination would be included in C Delay. Whether as B Delay or C Delay, the adjusted term would include this period of examination. Slip Op. at 8 (Newman, J., dissenting). The above summary is general in nature. Please see the Federal Circuit Opinion for complete details. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
May 10, 2019: USPTO Modifies PTA Procedures in view of Supernus [Updated] The USPTO has issued a Notice modifying its PTA Procedures in view of the recent Federal Circuit decision in Supernus v. Iancu, 2017-1357 (Fed. Cir. 2019). Supernus held that the USPTO is only authorized to reduce PTA for applicant delay equal to, and not exceeding, periods during which applicants fail to engage in reasonable efforts to conclude prosecution. Accordingly, in situations where USPTO regulations (see 37 CFR 1.704(c)) reduce PTA beyond actual failure-to-engage periods, patentees may seek correction under Supernus. See below, January 23, 2019, for further details of the Supernus holding. The Notice does not provide an ad hoc no-cost procedure for requesting PTA recalculation under Supernus, revise any PTA regulations, or change the USPTO's method of calculating PTA. Instead the Notice indicates patentees should seek Supernus-type corrections using the existing procedure in 37 CFR 1.705(b): A patentee who believes that the period of reduction provided for in 37 CFR 1.704(c)(8) (or any of 37 CFR 1.704(c)) exceeds the period of time during which the patentee failed to engage in reasonable efforts to conclude prosecution of the application because there is no identifiable effort the patentee could have undertaken to conclude prosecution of the underlying application may raise the issue in a timely request for reconsideration of the patent term adjustment under 37 CFR 1.705(b). The request for reconsideration must provide any relevant information, including factual support, which is not recorded in the USPTO’s PALM system to show that there was no identifiable effort the patentee could have undertaken to conclude prosecution of the underlying application during a portion of the period provided for in 37 CFR 1.704(c)(8) (or any of the periods set forth in 37 CFR 1.704(c)). For example, in a situation analogous to Supernus, the request for reconsideration must include the facts concerning how and when each of the documents contained in the information disclosure statement at issue were first cited by the USPTO or a foreign patent authority in a related or counterpart application. See 37 CFR 1.705(b)(2)(iv) (stating that a request for reconsideration must be accompanied by a statement of the facts involved, specifying “[a]ny circumstances during the prosecution of the application resulting in the patent that constitute a failure to engage in reasonable efforts to conclude processing or examination of such application as set forth in [37 CFR] 1.704”). The USPTO’s decision on any timely filed patentee request for reconsideration will apply the Federal Circuit's decision in Supernus in view of the information presented by the patentee. 84 FR at 20344 (paragraph breaks added and footnote omitted). While the Notice is directed to situations with "no identifiable effort the patentee could have undertaken to conclude prosecution," Supernus makes clear any PTA reduction exceeding actual failure-to-engage periods is improper. Furthermore, the Notice fails to take the opportunity to correct the basic unfairness of sometimes shifting years of USPTO delay to applicants for filing an IDS or supplemental reply. Like other PTA reductions, see 37 CFR 1.704(c)(3), (6), (9), and (10), Rule 1.704(c)(8) reductions should be limited to 4 months, the maximum allowable USPTO processing time under 35 USC 154(b)(1)(A)(ii). To the extent an IDS or supplemental reply results in a prosecution delay greater than 4 months, that delay is rightfully attributable to the USPTO. Patentees are reminded that the deadline to request reconsideration of PTA under 37 CFR 1.705(b) is within 2 months from the patent grant date, extendable by an additional 5 months pursuant to Rule 1.136(a). The 35 USC 154(b)(4)(A) deadline to seek correction by civil action is within 180 days after the date of the Director’s decision on applicant’s request for reconsideration. The above is general in nature. Please see the Notice for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
February 20, 2019: USPTO Weather-Related Closing The USPTO was closed on Wednesday, February 20, 2019 which is considered a "Federal holiday within the District of Columbia" under 35 USC § 21. Accordingly, where a 3-month 1.704(b) applicant response deadline falls on this date, the effective 3-month deadline for determining any PTA reduction is extended until Thursday, February 21, 2019. See ArQule v. Kappos, 793 F.Supp.2d 214 (D.D.C. 2011); MPEP § 2732. We have updated our calculator to automatically consider this closing in analyzing PTA rules when the Apply ArQule v. Kappos option (on by default) is selected on the Apply Term Rules tab. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
January 23, 2019: Federal Circuit Reverses PTA Reduction Exceeding Applicant Delay [Updated] Today, in Supernus v. Iancu, 2017-1357 (Fed. Cir. 2019), the US Court of Appeals for the Federal Circuit held that the USPTO exceeded its statutory authority by imposing a 646 day PTA reduction in US Patent No. 8,747,897 ("the '897 patent") for patentee's submission of an IDS after filing an RCE. The Court held that the PTA reduction went beyond the actual period during which the applicant failed to undertake reasonable efforts to conclude prosecution and thus exceeded the limitations set by the PTA Statute. 35 USC 154(b)(1) grants term adjustment to compensate patentees for certain USPTO prosecution delays. From this positive adjustment, section 154(b)(2)(C)(i) mandates that applicant delay be subtracted to compute the final PTA: The period of adjustment of the term of a patent ... shall be reduced by a period equal to the period of time during which the applicant failed to engage in reasonable efforts to conclude prosecution of the application. Except for one statutorily defined circumstance, 154(b)(2)(C)(ii), the USPTO is granted authority to establish what applicant conduct constitutes unreasonable delay by 35 USC 154(b)(2)(C)(iii): The Director shall prescribe regulations establishing the circumstances that constitute a failure of an applicant to engage in reasonable efforts to conclude processing or examination of an application. Pursuant to this authority, inter alia, the USPTO has deemed an IDS, filed after an RCE or other reply, to be a supplemental "other paper" generating a PTA reduction under 37 CFR 1.704(c)(8) (emphasis added): Circumstances that constitute a failure of the applicant to engage in reasonable efforts to conclude processing or examination of an application also include ... (8) Submission of a supplemental reply or other paper, other than a supplemental reply or other paper expressly requested by the examiner, after a reply has been filed, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the day after the date the initial reply was filed and ending on the date that the supplemental reply or other such paper was filed; The USPTO rationale for this rule is that an applicant submitting an IDS after filing an RCE interferes with the USPTO’s ability to process an application because the examiner may be forced to go back and review the application again. The reduction can be avoided, however, if the IDS is accompanied by a Rule 1.704(d) 30-day safe harbor statement. In the '897 patent prosecution, after a Final Rejection, Supernus filed an RCE on February 22, 2011. While awaiting a response from the USPTO, on August 21, 2012, the European Patent Office ("EPO") notified Supernus's European patent counsel of an Opposition citing 10 documents ("the Sandoz Opposition") filed in a related patent. 100 days later, on November 29, 2012, Supernus filed an IDS disclosing the Sandoz Opposition. Since this IDS was submitted after an RCE, and did not include a Safe Harbor statement, the USPTO reduced PTA by 646 days per Rule 1.704(c)(8) as shown: 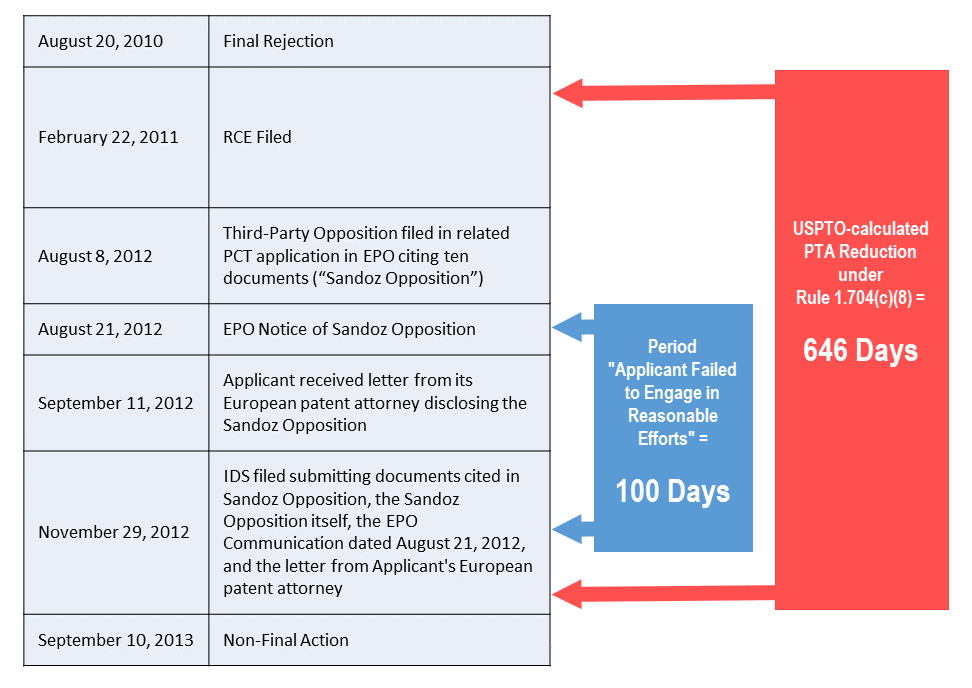 On appeal, Supernus argued inter alia that it did not fail to engage in "reasonable efforts" for all but the last 100 days of the reduction period because there was nothing it could have done to advance prosecution prior to the EPO notification of the Sandoz Opposition, on which the entire PTA reduction is predicated. Supernus was simply awaiting a USPTO response. The Court agreed: We agree with Supernus that there were no efforts that it could have taken in the period of time during the preceding 546 days ... beginning with the filing of the RCE/IDS on February 22, 2011, and ending on the date of the EPO notification, August 21, 2012. Nor does the USPTO contend that Supernus could have undertaken any "reasonable efforts" during the 546-day time period to conclude prosecution. To the contrary, the facts indicate that there was no action Supernus could have taken to advance prosecution of the patent during the 546-day period, particularly because the EPO notice of opposition did not yet exist. Slip Op. at 17. Accordingly, since the PTA Statute only allows for PTA reduction equal to, not exceeding, "applicant fail[ure] to engage" periods, the Court held the Rule 1.704(c)(8) reduction exceeded the USPTO's statutory authority: On the basis of the plain language of the statute, we hold that the USPTO may not count as applicant delay a period of time during which there was no action that the applicant could take to conclude prosecution of the patent.... * * * A plain reading of the statute shows that Congress imposed two limitations on the amount of time that the USPTO can use as applicant delay for purposes of reducing PTA. First, the statute expressly requires that any reduction to PTA be equal to the period of time during which an applicant fails to engage in reasonable efforts. Second, the statute expressly ties reduction of the PTA to the specific time period during which the applicant failed to engage in reasonable efforts. * * * ... Here, the USPTO's interpretation of the statute would unfairly penalize applicants, fail to incentivize applicants not to delay, and fail to protect applicants' full patent terms. The USPTO’s additional 546-day assessment as applicant delay is contrary to the plain meaning of the statute because the 646-day total reduction is not equal to a period of time during which Supernus failed to engage in reasonable efforts to conclude prosecution of the '897 patent. The USPTO's interpretation of the PTA statute applied in these circumstances exceeds the statutory limitations for PTA reduction and therefore, the USPTO actions are "in excess of statutory ... authority." Slip Op. at 18 (citation omitted). The Court distinguished the recent Gilead opinion, upholding a Rule 1.704(c)(8) PTA reduction, as involving different facts and a different legal question: Gilead simply did not address the precondition—failure to engage in reasonable efforts—at issue here.... Gilead focused on whether the statute required the applicant’s failure to take reasonable efforts to have resulted in actual delay, as opposed to the potential to cause delay, to count towards reduction of PTA.... The precise question in this case is whether the USPTO may reduce PTA by a period that exceeds the "time during which the applicant failed to engage in reasonable efforts to conclude prosecution." 35 USC 154(b)(2)(C)(i). Gilead did not decide that question. Slip Op. at 12. The Supernus holding is important for patentees as it makes clear the USPTO is only authorized to reduce PTA for applicant delay equal to, and not exceeding, periods during which the applicant failed to engage in reasonable efforts to conclude prosecution. Sometimes, in particular prosecution scenarios, Rule 1.704 PTA reductions can produce unfair term penalties. Supernus should help patentees seek correction in these circumstances. The above overview is general in nature. Please see the Federal Circuit Opinion for complete details. In addition, further rules and guidance may be issued by the USPTO. Patentees are advised to recheck PTA calculations in light of Supernus to determine if any Rule 1.704(c)(8) IDS reductions (or any other Rule 1.704 PTA reductions) exceed the actual applicant delay and to seek correction in appropriate cases. We will be updating our calculator with rule warnings on this issue, and to provide an updated rule on the Apply Term Rules tab. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
January 14, 2019: USPTO Weather-Related Closing The USPTO was closed on Monday, January 14, 2019 which is considered a "Federal holiday within the District of Columbia" under 35 USC § 21. Accordingly, where a 3-month 1.704(b) applicant response deadline falls on this date, the effective 3-month deadline for determining any PTA reduction is extended until Tuesday, January 15, 2019. See ArQule v. Kappos, 793 F.Supp.2d 214 (D.D.C. 2011); MPEP § 2732. We have updated our calculator to automatically consider this closing in analyzing PTA rules when the Apply ArQule v. Kappos option (on by default) is selected on the Apply Term Rules tab. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
December 24, 2018: USPTO Holiday Closing The USPTO was closed on Monday, December 24, 2018 and Tuesday, December 25, 2018, which are considered "Federal holiday[s] within the District of Columbia" under 35 USC § 21. Accordingly, where a 3-month 1.704(b) applicant response deadline falls on these days, the effective 3-month deadline for determining any PTA reduction is extended until Wednesday, December 26, 2018. See ArQule v. Kappos, 793 F.Supp.2d 214 (D.D.C. 2011); MPEP § 2732. We have updated our calculator to automatically consider this closing in analyzing PTA rules when the Apply ArQule v. Kappos option (on by default) is selected on the Apply Term Rules tab. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
December 05, 2018: USPTO Closing Affects PTA In view of the National Day of Mourning, the USPTO was closed on Wednesday, December 5, 2018, which will be considered a "Federal holiday within the District of Columbia" under 35 USC § 21. Accordingly, where a 3-month 1.704(b) applicant response deadline falls on this date, the effective 3-month deadline for determining any PTA reduction is extended to Thursday, December 6, 2018. See ArQule v. Kappos, 793 F.Supp.2d 214 (D.D.C. 2011); MPEP § 2732. We have updated our calculator to automatically consider this closing in analyzing PTA rules when the Apply ArQule v. Kappos option (on by default) is selected on the Apply Term Rules tab. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
November 02, 2018: USPTO Implements Interim Procedure to Request PTA Recalculation for IDS Safe Harbor Statements [Updated] If an applicant files an Information Disclosure Statement (IDS) at certain times during prosecution, the filing may be considered applicant delay generating a PTA reduction. See 37 CFR 1.704(c)(6), (8), (9), (10), and (12). Common examples include filing an IDS after replying to an Office action or after a notice of allowance. Id. In appropriate circumstances, applicants can avoid this PTA reduction by filing the IDS accompanied by a 30-day Safe Harbor Statement under 37 CFR 1.704(d). When calculating PTA, however, the USPTO automated computer program typically fails to account for Safe Harbor Statements, thus improperly reducing PTA for some patents and requiring applicants to seek manual correction by filing an Application for PTA under 37 CFR 1.705(b) and paying a petition fee. In light of this issue, the USPTO has implemented a simplified Interim Procedure for requesting PTA recalculation and is making other improvements: When the USPTO receives a Form PTO/SB/134 request for recalculation, the Office of Petitions will manually review the PTA calculation under 37 CFR 1.702 through 1.704 (note that these rules cover all USPTO and applicant delay). The patentee will be given one opportunity to respond to the recalculation within two months (non-extendable) of the recalculation mail date. If the patentee fails to respond to the recalculation, the USPTO will sua sponte issue a certificate of correction if needed to correct the PTA printed on the patent. If the patentee responds to the recalculation by requesting changes not related to the Safe Harbor Statement, the patentee must comply with the requirements of 37 CFR 1.705(b)(1) and (2). If the patentee files a timely response and the USPTO maintains its recalculation, the USPTO will issue a decision confirming its recalculation pursuant to 35 USC 154(b)(3)(B)(ii), and this decision is the appealable Director’s decision under 35 USC 154(b)(4). The USPTO’s initial PTA recalculation under the Interim Procedure, however, is not the appealable Director’s decision under 35 USC 154(b)(4). The above overview is general in nature. Please see 83 FR 55102 (Nov. 2, 2018), Form PTO/SB/133, and Form PTO/SB/134 for complete details. When calculating PTA, patentees are advised to manually check Information Disclosure Statement filings for Safe Harbor Statements and seek correction in appropriate cases. In our calculator, users can indicate a Safe Harbor Statement using the appropriate checkbox on the Apply Term Rules tab. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
April 26, 2018: EDVA Upholds USPTO B-Delay Calculation In Mayo Clinic v. Iancu, 1:17-cv-1153 (E.D. Va. 2018), the US District Court for the Eastern District of Virginia granted summary judgment upholding a USPTO PTA determination of B-Delay. B-Delay is a type of PTA under 35 USC 154(b)(1)(B) accruing if the USPTO fails to issue a patent within three years. B-Delay does not include inter alia "any time consumed by continued examination of the application requested by the applicant under section 132(b)" or "any time consumed by a [derivation or interference] proceeding under section 135(a)". 35 USC 154(b)(1)(B)(i) and (ii). In Mayo, per its usual practice, the USPTO determined the entire period from an RCE filing until the mailing of a notice of allowance to be "time consumed by continued examination" excluded from B-Delay. See 37 CFR 1.703(b)(1). In contrast, Mayo asserted that "continued examination" ended earlier when an interference was declared in its application. Mayo argued that a declaration of interference is analogous to allowance as claims are otherwise "deemed allowable" by the examiner before the interference is declared. Since the interference proceeding was separately excluded from B-Delay per 35 USC 154(b)(1)(B)(ii), left at issue was whether the time after termination of the interference until the notice of allowance should have been excluded from B-Delay. The Court rejected Mayo's contention, holding that an interference declaration does not end continued examination for PTA purposes: In the end, Mayo's arguments fail because Federal Circuit precedent makes clear that "time consumed by continued examination of the application requested by the applicant" includes time up until "allowance"--that is, when the notice of allowance is mailed, prosecution is closed, and further examination on the merits does not occur absent a special reopening. Novartis, 740 F.3d at 602. A declaration of interference is not allowance; after interference proceedings conclude, jurisdiction automatically returns to the examiner, and the examiner is required to conduct additional examination by updating prior art searches. As such, prosecution remains open at this point, further examination is routine, not exceptional, and this time is properly considered "time consumed by continued examination of the application requested by the applicant" pursuant to § 154(b)(1)(B). Slip Op. at 16. The above summary is general in nature. Please see the District Court Opinion for complete details. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
March 12, 2018: EDVA Upholds USPTO PTA Reduction for Applicant Delay [Updated] In Intra-Cellular Therapies, Inc. v. Matal, 1:17-cv-00776 (E.D. Va. 2018), the US District Court for the Eastern District of Virginia granted summary judgment upholding the USPTO's PTA reduction for applicant's late response to a final Office action. 35 USC 154(b)(2)(C)(i) mandates that applicant delay be subtracted from USPTO delay to compute PTA. One such applicant delay is taking more than 3 months to respond to a USPTO notice. 35 USC 154(b)(2)(C) provides: The period of adjustment of the term of a patent ... shall be reduced by a period equal to the period of time during which the applicant failed to engage in reasonable efforts to conclude prosecution of the application.... [A]n applicant shall be deemed to have failed to engage in reasonable efforts to conclude processing or examination of an application for the cumulative total of any periods of time in excess of 3 months that are taken to respond to a notice from the Office making any rejection, objection, argument, or other request, measuring such 3- month period from the date the notice was given or mailed to the applicant. 37 CFR 1.704(b), promulgated by the USPTO under its authority to "prescribe regulations establishing the circumstances that constitute a failure of an applicant to engage in reasonable efforts," see 35 USC 154(b)(2)(C)(iii), also makes this requirement: ... [A]n applicant shall be deemed to have failed to engage in reasonable efforts to conclude processing or examination of an application for the cumulative total of any periods of time in excess of three months that are taken to reply to any notice or action by the Office making any rejection, objection, argument, or other request, measuring such three-month period from the date the notice or action was mailed or given to the applicant, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the day after the date that is three months after the date of mailing or transmission of the Office communication notifying the applicant of the rejection, objection, argument, or other request and ending on the date the reply was filed.... The Intra-Cellular dispute involves the following prosecution events: | April 17, 2013 | Final Office Action | Rejects or objects to all outstanding claims. | | July 17, 2013 | Amendment and Response after Final | Amends claims, presents arguments, and adds one new claim. | | July 26, 2013 | Advisory Action | Indicates submission overcomes some, but not all, rejections and objections; new claim would be allowable if submitted separately; and the time period continues to run from mailing date of final rejection. Enters amendments for purposes of appeal. | | August 7, 2013 | Amendment and Response to Advisory Action | Cancels, further amends, and adds claims overcoming all outstanding objections and rejections. | | August 20, 2013 | Notice of Allowance | Allows remaining claims. | The USPTO assessed 21 days of applicant delay, concluding that applicant's first submission on July 17, 2013 was not a proper reply under 37 CFR 1.113(c) and thus applicant had failed to engage in reasonable efforts to conclude prosecution until its second submission on August 7, 2013. In contrast, the applicant argued the USPTO erred in equating a "reply" under 37 CFR 1.704(b) with a "reply" under 37 CFR 1.113, and that no PTA reduction is warranted because its first submission substantially advanced prosecution and amounted to "reasonable efforts to conclude prosecution." Applicant further argued that, by entering the Amendment, the USPTO effectively conceded the first response was valid. After finding the plain language of the Statute did not resolve the issue, the Court upheld the USPTO determination based on the regulations, finding that 37 CFR 1.704(b) and 37 CFR 1.113(c) are indeed interconnected: [T]he PTA calculation explicitly relies in part on whether a "reply" complies with § 1.113(c), and thus a "reply" under § 1.704(b) is therefore inextricably linked with § 1.113(c), and a "reply" under § 1.704(b) must meet the requirements of § 1.113(c).... Looking then to § 1.113(c), that regulation states that a "[r]eply to a final rejection or action must include cancellation of, or appeal from the rejection of, each rejected claim. If any claim stands allowed, the reply to a final rejection or action must comply with any requirements or objections as to form." 37 CFR § 1.113(c). In other words, a proper reply under § 1.113(c) to a final Office action must resolve all rejections or objections, otherwise it does not stop the three-month clock for assessing applicant delay. Because Plaintiff's July 17, 2013 submission did not cancel all of the rejected claims or remove all of the outstanding objections identified in the final Office action, the USPTO determined that the submission was not a proper reply under § 1.113(c), and was therefore not a "reply" as defined in § 1.703(a)(3) and as incorporated into § 1.704(b). * * * The USPTO determined that Plaintiff's July 17, 2013 submission was not a valid reply under § 1.703(b), and therefore constituted a "fail[ure] to engage in reasonable efforts to conclude processing or examination" under § 1.704(b). This Court finds that the USPTO's determination was based on a permissible construction of the relevant statutes, and therefore should be afforded deference under Chevron. Furthermore, this Court finds that the USPTO did not concede that the July 17, 2013 submission was a valid reply in compliance with § 1.113(c) by entering the amendments presented in the submission. Accordingly, the USPTO's Final Decision that the July 17, 2013 submission did not stop the clock under § 1.703(b) for accruing applicant delay was not arbitrary and capricious, and should be affirmed. Slip Op. at 12. The above overview is general in nature. Please see the District Court opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
February 07, 2018: Federal Circuit Upholds USPTO A-Delay Calculation [Updated] In Actelion v. Matal, 2017-1238 (Fed. Cir. 2018), the US Court of Appeals for the Federal Circuit upheld the USPTO's PTA calculation of A-Delay for the subject patent. This ruling validates the USPTO's long-standing implementation of several technical aspects of 35 USC 154(b)(1)(A)(i) (14-month USPTO first action deadline) as applied to national stage applications. A-Delay is a type of PTA accruing under 35 USC 154(b)(1)(A) if the USPTO fails to act by certain specific examination deadlines. The deadline at issue in Actelion requires the USPTO to provide a first action (e.g., a section 132 Office action or notice of allowance) in 14 months. See 35 USC 154(b)(1)(A)(i). For national stage applications, prior to the 2013 AIA Technical Corrections Act ("TCA"), the 14-month deadline began to run upon fulfillment of the 371 requirements. For applications subject to TCA, the 14-month deadline begins to run upon national stage commencement: | 14-Month USPTO First Action Deadline in National Stage Applications | | Pre-TCA Statute | Post-TCA (Current) Statute | | [I]f the issue of an original patent is delayed due to the failure of the [USPTO] to (i) provide at least one of the notifications under section 132 of this title or a notice of allowance under section 151 of this title not later than 14 months after … the date on which an international application fulfilled the requirements of section 371 … the term of the patent shall be extended 1 day for each day after the end of the period … until the action … is taken. | [I]f the issue of an original patent is delayed due to the failure of the [USPTO] to (i) provide at least one of the notifications under section 132 or a notice of allowance under section 151 not later than 14 months after … the date of commencement of the national stage under section 371 in an international application … the term of the patent shall be extended 1 day for each day after the end of the period … until the action … is taken. | | 35 USC 154(b)(1)(A)(i)(II) (pre-TCA) (emphasis added) | 35 USC 154(b)(1)(A)(i)(II) (emphasis added) |
The Actelion ruling addresses several issues relating to both the pre-TCA and current versions of 35 USC 154(b)(1)(A)(i)(II) for national stage applications: (1) Applicability Date of TCA Amendment Although the parties disputed which version of 35 USC 154(b)(1)(A)(i)(II) applies to the subject patent, the Actelion Court did not reach this issue, finding the same result under both versions. Nonetheless, confirmation of the USPTO's interpretation of the TCA applicability date is an important issue and may arise in future litigation. See our discussion below on January 15, 2013 and April 3, 2013 for more information. (2) 371(f) "Express Request" for National Stage Commencement While the national stage commences upon expiration of the 30-month time limit, see 35 USC 371(b), an applicant may initiate earlier commencement by making an "express request" under 35 USC 371(f). The USPTO provides a checkbox on PTO Form 1390 (a transmittal letter form) to make this request. Although it used the form and did not check the box, Actelion nonetheless contended it made a 371(f) express request via its remark that it "earnestly solicits early examination and allowance of these claims." The Court rejected this contention: … Using the PTO form may be optional, and, as Actelion contends, there may be other ways to communicate to the PTO an "express request" pursuant to § 371(f). However, neither the fact that using the PTO forms may be optional nor the availability of other § 371(f)-compliant means of making an express request excuses an applicant's failure to make its intention clear…. Even viewed most favorably to Actelion, the casual "solicits early examination" language with no reference to § 371(f), the PCT, or the national stage, when combined with the unchecked box 3 of its completed PTO Form 1390, was, if not an express election not to commence the national stage early, at least an inconsistent or ambivalent request. We therefore find no error in the PTO's determination that Actelion's submission of PTO Form 1390 with box 3 unchecked, and its precatory solicitation remark having no operative consequence, did not amount to an express request pursuant to § 371(f). Slip Op. at 12 (citation omitted). (3) 371 Fulfillment Date under Pre-TCA Statute Where an applicant does not expressly request commencement of the national stage under 35 USC 371(f), an application may fulfill the requirements of 371(c) prior to the national stage commencing under 371(b) (i.e., upon the expiration of the 30 month time limit). For its application, Actelion argued the earlier 371(c) fulfillment date, rather than the later 371(b) commencement date, constitutes the date it "fulfilled the requirements of section 371" for purposes of pre-TCA 35 USC 154(b)(1)(A)(i)(II), thus starting the USPTO's 14-month deadline earlier. The Court rejected Actelion's contention, upholding the USPTO's long-standing view that its 14-month deadline cannot begin prior to the national stage commencement date, see MPEP 2731 (2018) at 2700-18. The Court held: The PTO responds that the pre-TCA "requirements of section 371" include the requirements under § 371(b) and (f), and that, under the PCT, the national stage of an international application cannot commence prior to the expiration of the exclusive 30-month international processing period unless early examination is expressly requested by the applicant pursuant to § 371(f).... We agree with the PTO that, under either the pre- or post-TCA version of § 154(b)(1)(A)(i)(II), the A Delay calculation must be based on the date on which the entirety of § 371 is complied with, including § 371(b) and (f). As such, we conclude that under either pre- or post-TCA law, Actelion was required to comply with the "express request" provision of § 371(f) if it wished to commence the national stage before the expiration date provided in § 371(b). Slip Op. at 10. (4) Effect of Holiday on National Stage Commencement under 35 USC 371(b) (Expiration of 30-Month Time Limit) For Actelion's application, the end of the 30-month time limit under 371(b) fell on Martin Luther King, Jr. Day, a national holiday. Rejecting Actelion's arguments, the Court upheld the USPTO's position that, since the 30-month time limit of 371(b) fell on a holiday, the national stage commenced on the next business day: Actelion's "no holiday exception" argument, similar to its pre-TCA statutory argument, is premised on the assumption that any time period of inaction that is not attributable to the applicant should inure to the applicant's benefit. As such, Actelion emphasizes its alleged lack of fault during the time periods in question. However, by the same logic, inaction on a holiday is also not attributable to the PTO. Although the PTA statutes do serve a remedial purpose of restoring patent term lost during prosecution of an application, they only restore "undue delays in patent examination caused by the PTO" as provided by Congress. Pfizer, 811 F.3d at 468 (emphasis added). We find no error in the PTO's determination that the national stage for the '675 patent did not commence until the next workday after the 30-month date that fell on a federal holiday. Slip Op. at 14. The above overview is general in nature. Please see the Federal Circuit opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
January 19, 2018: EDVA Overturns USPTO B-Delay Calculation In ARIAD Pharma v. Matal, 1:17-cv-733 (E.D. Va. 2018), the US District Court for the Eastern District of Virginia granted summary judgment overturning a USPTO PTA determination of B-Delay. The ruling is predicated on a somewhat uncommon prosecution circumstance--a withdrawn abandonment after an RCE filing--and thus its effect may be limited. B-Delay is a type of PTA under 35 USC 154(b)(1)(B) accruing if the USPTO fails to issue a patent within three years. B-Delay does not include inter alia "any time consumed by continued examination of the application requested by the applicant under section 132(b)." 35 USC 154(b)(1)(B)(i). In the ARIAD prosecution, in response to a final rejection, the applicant filed an RCE, which normally begins the B-Delay exclusion for time consumed by continued examination. See 37 CFR 1.703(b)(1). Instead of acting on the RCE, however, the USPTO erroneously issued a notice of abandonment suggesting that no response had been filed. The applicant then petitioned and the USPTO withdrew the abandonment since Office records indicated a reply had in fact been received. Agreeing with ARIAD, the District Court granted summary judgment holding that the erroneous abandonment, although after the RCE, was not "time consumed by continued examination" excluded from B-Delay: The central dispute in this case is whether the PTO's exclusion of time during which the PTO erroneously considered ARIAD's application to be abandoned comports with the statutory requirement that "B Delay" should exclude only "time consumed by continued examination."… [I]t is important to note that Congress did not use the phrase--"time after the applicant filed a request for continued examination" or "time attributable to a request for continued examination" in the statutory text. Instead, Congress chose to draft the provision as "any time consumed by continued examination of the application requested by the applicant." …The ordinary [Webster's Dictionary] meaning of "consumed by" is "used in the course of." Time cannot possibly be used in the course of continued examination where, as here, the PTO erroneously determines the application is abandoned and does not believe it has even received an RCE.... Accordingly, the PTO's exclusion of time when the PTO erroneously considered an application abandoned from ARIAD's "B Delay" calculation is inconsistent with the plain text of the statute. * * * ... Put simply, in computing “time consumed by continued examination,” the PTO should not include time when the PTO was plainly not conducting continued examination, but instead negligently concluded that the patent application had been abandoned. Thus, plaintiff is entitled to judgment as a matter of law. Slip Op. at 7 (footnote and citations omitted). The above summary is general in nature. Please see the District Court opinion for complete details. At this time, to apply this holding in our PTA calculator, you should manually adjust PTA rules on the Apply Term Rules tab by, e.g., moving the Exclusion for Continued Examination rule. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
December 05, 2017: Oral Argument Heard in Supernus Federal Circuit Appeal Yesterday, Judges Dyk, Schall, and Reyna of the US Court of Appeals for the Federal Circuit heard oral argument on Supernus's appeal of the EDVA decision upholding the USPTO’s 646 day PTA reduction under 37 CFR 1.704(c)(8) for patentee's filing of a supplemental paper, an IDS, after filing an RCE. A recording of the oral argument is available on the Federal Circuit website. Briefs and further case details are available below. The panel was clearly troubled by the USPTO charging the patentee with failing to engage in reasonable efforts to conclude prosecution for an extended period in which patentee was waiting for an Office action, had no options to act, and did nothing improper. Nonetheless Judge Schall questioned the practicality of the USPTO making case-by-case reasonableness determinations versus a bright line rule for all cases. If the Court invalidates the portion of the regulation delineating the magnitude of the PTA reduction, however, we believe no such individualized determinations will be required. Instead, the USPTO would likely revise its regulation (and update its computerized algorithm) to set a PTA reduction more closely corresponding to the actual delay. Some possible revisions are discussed below. We expect a ruling will be issued in the first quarter of 2018. In the meantime, patentees are advised to consider the possibility of a future rule change in light of this appeal, and to recheck USPTO-calculated PTA and seek correction in appropriate cases. The above is general in nature. Please see the briefs and legal authorities for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
June 10, 2017: Briefing Completed in Supernus Federal Circuit Appeal Briefing has been completed in Supernus's appeal of the EDVA decision upholding the USPTO’s 646 day PTA reduction for patentee's filing of a supplemental paper (an IDS after an RCE). This appeal presents strong arguments that the magnitude of the 37 CFR 1.704(c)(8) PTA reduction is contrary to the 35 USC 154(b)(2)(C)(i) requirement that the reduction equal--not exceed--the period of applicant delay. As Rule 1.704(c)(8) is written, many patentees receive a punitive term reduction for filing a supplemental amendment or other paper after responding to an Office action. Briefs are available here: Please see our discussion below for more details on this case. Patentees are advised to consider the possibility of a future rule change in light of this appeal, and to recheck USPTO-calculated PTA and seek correction in appropriate cases. The deadline to request reconsideration of PTA under 37 CFR 1.705(b) is within 2 months from the patent grant date, extendable by an additional 5 months pursuant to Rule 1.136(a). The 35 USC 154(b)(4)(A) deadline to seek correction by civil action is within 180 days after the date of the Director’s decision on applicant’s request for reconsideration. The above is general in nature. Please see the briefs and legal authorities for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
November 15, 2016: EDVA Upholds Unfair PTA Penalty for IDS after RCE [Updated] In Supernus v. Lee, 1:16-cv-342 (E.D. Va. 2016), the US District Court for the Eastern District of Virginia granted summary judgment upholding the USPTO’s 646 day PTA reduction for patentee’s filing of an IDS after an RCE. While some aspects of the disputed PTA rule were recently upheld by the Federal Circuit in Gilead Sciences, Inc. v. Lee, 778 F.3d 1341 (Fed. Cir. 2015), the Supernus facts more clearly demonstrate a logical flaw in the portion of the USPTO regulation delineating the magnitude of the PTA reduction, offering the Federal Circuit an opportunity to revisit the regulation on appeal. The American Inventor’s Protection Act (AIPA) grants term adjustment to compensate patentees for certain USPTO prosecution delays as set forth in 35 USC 154(b)(1). From this positive adjustment, 35 USC 154(b)(2)(C)(i) mandates that applicant delay be subtracted to compute the final PTA: The period of adjustment of the term of a patent ... shall be reduced by a period equal to the period of time during which the applicant failed to engage in reasonable efforts to conclude prosecution of the application. Except for one statutorily defined circumstance, 154(b)(2)(C)(ii), the USPTO is granted authority to establish what applicant conduct constitutes such unreasonable delay by 35 USC 154(b)(2)(C)(iii): The Director shall prescribe regulations establishing the circumstances that constitute a failure of an applicant to engage in reasonable efforts to conclude processing or examination of an application. Pursuant to this authority, the USPTO has deemed an IDS, filed after an RCE or other reply, to be a supplemental “other paper” generating a PTA reduction under 37 CFR 1.704(c)(8) (emphasis added): Circumstances that constitute a failure of the applicant to engage in reasonable efforts to conclude processing or examination of an application also include ... (8) Submission of a supplemental reply or other paper, other than a supplemental reply or other paper expressly requested by the examiner, after a reply has been filed, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the day after the date the initial reply was filed and ending on the date that the supplemental reply or other such paper was filed; The general rationale is that an IDS, when filed after an applicant reply, may delay prosecution since the examiner may already be working on a response, and may be forced to go back and re-review the application, while still trying to meet timeliness obligations under 35 USC 154. Per the regulation, the PTA reduction can be avoided if the applicant provides a Rule 1.704(d) 30-day safe harbor statement, but Supernus did not so its IDS was subject to the rule. For the Supernus patent, Rule 1.704(c)(8) delineates a 646 days PTA reduction based on the following prosecution history events: 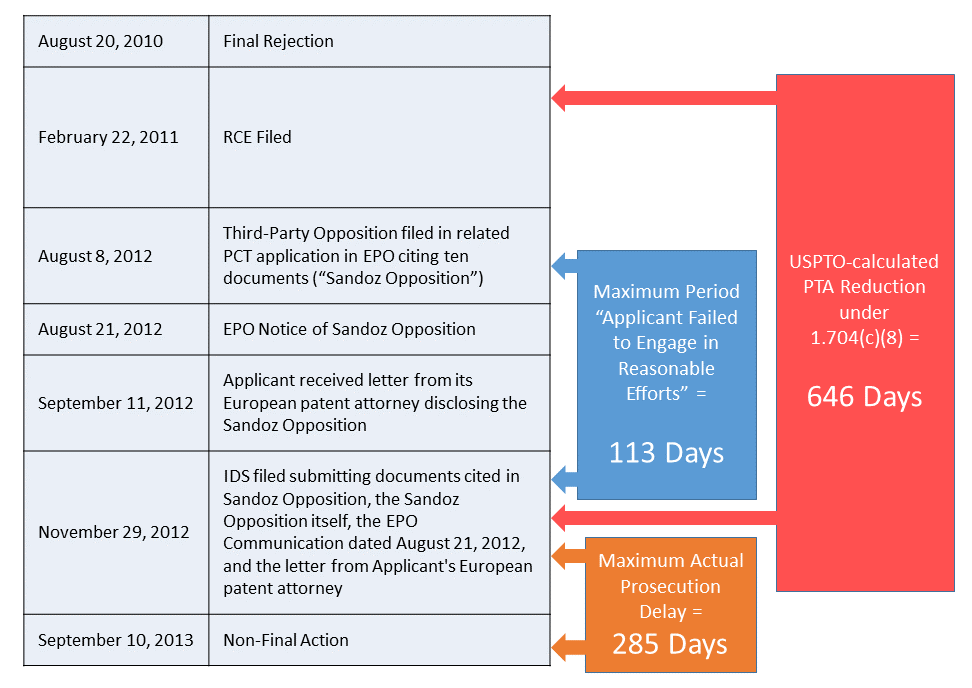 As specified in Rule 1.704(c)(8), the reduction runs from the from the RCE filing date (applicant's initial reply) until the IDS filing date (applicant's supplemental reply or other paper), a period of 646 days as shown above. This 646 day reduction period, however, fails to corresponds to the "period of time during which the applicant failed to engage in reasonable efforts to conclude prosecution" as required by 35 USC 154(b)(2)(C)(i). The USPTO begins charging Supernus with failing to engage in reasonable efforts for filing an IDS based on the Sandoz Opposition 18 months before the Sandoz Opposition even came into existence. Even assuming arguendo that Supernus should have filed its IDS on the same day of the Sandoz Opposition, it did so only 113 days later (only 83 days after the 1.704(d) safe harbor deadline). A failure to engage period of 646 days, including time before the subject Opposition even existed, is illogical. Moreover, the notion that Supernus caused 646 days of prosecution delay by filing an IDS is unfair. How can an IDS cause that much delay? Most of the delay is actually attributable to the USPTO's 21-month failure to act even before the IDS was filed. Even assuming arguendo that the examiner was about to issue an Office action on the same day as the IDS, but pulled and reworked the Office action because of the IDS, the maximum actual prosecution delay is 285 days, the period after the IDS as shown above. Of course, if it takes an examiner 285 days to process an IDS, much of that delay is rightfully attributable to the USPTO, not the applicant. Relying on Gilead, the Supernus Court refused to consider such specifics of the Supernus IDS: The PTA statute does not require the PTO to make any sort of particularized determination of PTA, and instead directs the PTO to promulgate rules of general applicability that address the circumstances that will lead to a deduction of PTA. § 1.704(c)(8) is one of these rules. The Federal Circuit in Gilead upheld the reasonableness of the PTO's interpretation of 35 USC § 154 by finding that § 1.704(c)(8) is not arbitrary, capricious, or otherwise not in accordance with law. Accordingly, because § 1.704(c)(8) applies squarely to this case, Plaintiff's claim must fail as a matter of law. This ignores the larger issue, however, that the 1.704(c)(8) reduction period is not properly drafted to correspond to the "period of time during which the applicant failed to engage in reasonable efforts" and ignores delays caused by the USPTO. Although not addressed in Supernus, note that the USPTO is charged with "establishing the circumstances that constitute a failure of an applicant to engage in reasonable efforts" (e.g., filing an IDS after a reply), not defining the magnitude of the reduction (i.e., "period of time during which the applicant failed to engage in reasonable efforts") associated with such circumstances. Compare 35 USC 154(b)(2)(C)(iii) with 35 USC 154(b)(2)(C)(i). A fairer PTA reduction would perhaps run from the IDS filing date until the next Office action or allowance, and would definitely be limited to a maximum penalty such as four months. The same maximum is reflected in other USPTO-defined applicant delays, see 37 CFR 1.704(c)(3), (6), (9), and (10), and would account for the fact that excessive processing time is rightfully attributable to the USPTO. The above overview is general in nature. Please see the District Court opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
October 31, 2016: EDVA Upholds USPTO’s Implementation of 14-Month Rule for National Stage Applications In Actelion v. Lee, 1:16-cv-304 (E.D. Va. 2016), the US District Court for the Eastern District of Virginia granted summary judgment upholding the USPTO’s PTA calculation of A-Delay for the subject patent. This ruling validates the USPTO's implementation of several technical aspects of both the current and previous versions of 35 USC 154(b)(1)(A)(i) as applied to national stage applications. A-Delay is a type of PTA accruing under 35 USC 154(b)(1)(A) if the USPTO fails to act by certain examination deadlines. The deadline at issue in Actelion requires the USPTO to provide a first action (e.g., a section 132 Office action or notice of allowance) in 14 months. See 35 USC 154(b)(1)(A)(i). For national stage applications, the starting date of this deadline was revised in 2013 by the AIA Technical Corrections Act (AIA TCA): | 14-Month USPTO First Action Deadline in National Stage Applications | | Pre-AIA TCA Statute | Current Statute | | [I]f the issue of an original patent is delayed due to the failure of the [USPTO] to (i) provide at least one of the notifications under section 132 of this title or a notice of allowance under section 151 of this title not later than 14 months after … the date on which an international application fulfilled the requirements of section 371 … the term of the patent shall be extended 1 day for each day after the end of the period … until the action … is taken. | [I]f the issue of an original patent is delayed due to the failure of the [USPTO] to (i) provide at least one of the notifications under section 132 or a notice of allowance under section 151 not later than 14 months after … the date of commencement of the national stage under section 371 in an international application … the term of the patent shall be extended 1 day for each day after the end of the period … until the action … is taken. | | 35 USC 154(b)(1)(A)(i)(II) (pre-AIA TCA) (emphasis added) | 35 USC 154(b)(1)(A)(i)(II) (emphasis added) |
The Actelion ruling encompasses issues under both the pre-AIA TCA and current versions of 35 USC 154(b)(1)(A)(i) as applied to national stage applications: (1) Applicability Date of AIA TCA Amendment The parties disputed which version of 35 USC 154(b)(1)(A)(i)(II) applies to the subject patent, granted in 2014, but having a national stage commencement date in 2012, and a PCT filing date in 2010. Per its interpretation of the AIA TCA, the USPTO asserts the amendment applies since the patent was granted on or after January 14, 2013. See Interim Final Rule, Revisions to Patent Term Adjustment, 78 FR 19416 (Apr.1, 2013); Final Rule, Revisions To Implement the Patent Term Adjustment Provisions of the Leahy-Smith America Invents Act Technical Corrections Act, 79 FR 27755 (May 15, 2014). Since the Actelion Court held that the PTA is the same under both versions, however, it did not reach this issue. Nonetheless, confirmation of the USPTO's interpretation of the AIA TCA applicability date is an important point and may arise in future litigation. See our discussion below on January 15, 2013 and April 3, 2013 for more information. (2) 371 Fulfillment Date under Pre-AIA Statute Where an applicant does not expressly request commencement of the national stage under 35 USC 371(f), an application may fulfill the requirements of 371(c) prior to the national stage commencing under 371(b) (i.e., upon the expiration of the 30 month time limit). For its application, Actelion argued the 371(c) fulfillment date, rather than the later 371(b) commencement date, constitutes the date it "fulfilled the requirements of section 371" for purposes of pre-AIA TCA 35 USC 154(b)(1)(A)(i)(II), thus starting the USPTO's 14-month deadline. The Court rejected Actelion's contention, upholding the USPTO’s long-standing view that its 14-month deadline cannot begin prior to the national stage commencement date, see MPEP 2731 (2010) at 2700-12, since the USPTO time limit to act would begin even before the application was pending before the Office. The Court held: As [the USPTO] convincingly argues, [Actelion's] characterization of the pre-AIA TCA A Delay calculation would incoherently permit a filer to begin accruing A-Delay on the day of filing the application in compliance with only § 371(c) based on the PTO's purported delay in reviewing the application, even if the filer refused early examination under § 371(f), because the PTO would be prohibited by statute from reviewing the application until the end of the 30 month period as required by § 371(b). (3) Effect of Weekend or Holiday on National Stage Commencement under 35 USC 371(b) (Expiration of 30-Month Time Limit) For Actelion's application, the end of the 30-month time limit under 371(b) fell on Martin Luther King, Jr. Day, a national holiday. Rejecting Actelion's arguments, the Court upheld the USPTO's position that, since the 30-month time limit of 371(b) fell on a weekend or holiday, the national stage commenced on the next business day: While [Actelion] completed in advance the actions required under PCT Art. 22(1) as a necessary condition to [35] USC § 371(b); the statutory scheme contemplates that a filer need not take an action until the expiration date. Because a filer can take actions on the expiration date, the PTO must be available to receive and take action on such filings-which is not possible on a weekend or federal holiday-precisely the consideration which [35 USC] § 21(b) is intended to ameliorate. Accordingly, [the USPTO’s] decision to apply the weekend/holiday exception is consistent with the interpretation of similar statutory provisions and is not a clear error of judgment. The above overview is general in nature. Please see the District Court opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
May 29, 2016: EDVA Dismisses Another B-Delay PTA Challenge In Maass v. Lee, 1:16-cv-66 (E.D. Va. 2016), the US District Court for the Eastern District of Virginia dismissed another pro se challenge that, under various statutory and Constitutional grounds, time consumed by RCE's should not be excluded from B-Delay as set forth in 35 USC 154(b)(1)(B). The Court squarely rejected this contention as contrary to the PTA statute: [P]laintiff's challenge to the PTO's B-Delay calculation must be dismissed because [35 USC] 154(b)(1)(B)(i) makes clear that, without exception, the calculation of B-Delay does not include "any time consumed by continued examination of the application requested by the applicant under [§] 132(b)." ... [T]here is no statutory or constitutional basis for plaintiff's claim that the PTO erred in its PTA calculation ... of B-Delay ...." This conclusion is consistent with the recent Singhal v. Lee holding discussed below. It is also consistent with how the USPTO, and most applicants, typically calculate PTA. Remaining in the Maass action is a separate challenge, the details of which were not reached by the Court, concerning the amount of applicant delay associated with the filing of a supplemental amendment and applicant delay accrued during continued examination. Under a joint motion, these issues have been remanded to the USPTO for reconsideration. The above overview is general in nature. Please see the District Court opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
April 26, 2016: Printing Error Causes USPTO to Understate PTA For some patents issuing on March 22, 2016, the USPTO printed the incorrect PTA value on the face of the patent, understating PTA by 1 to 7 days compared to the USPTO-calculated value shown in the PAIR system. Since the PTA value printed on the patent is the USPTO’s "official" determination of PTA, affected patentees should seek correction to ensure the proper term in appropriate cases. See MPEP 2733 at 2700-39; Final Rule, Revisions To Implement the Patent Term Adjustment Provisions of the Leahy-Smith America Invents Act Technical Corrections Act, 79 FR 27755 at 27757 (May 15, 2014). The USPTO has notified impacted patentees of this error (see example notification letter) suggesting several possible courses of action: - If the patentee considers the PTA displayed in PAIR to be correct, patentee may request a certificate of correction under 37 CFR 1.322 within two months of the notification letter (no extension of time is available). No fee is required.
- If the patentee believes the correct PTA is an amount different than in PAIR or printed on the patent, patentee may file a Request for Reconsideration of PTA under 37 CFR 1.705 within two months of patent issue date, extendable by an additional five months pursuant to Rule 1.136(a).
- Alternatively, if the patentee believes he is entitled to less PTA than printed on the patent, patentee may disclose the error in a letter to the USPTO that will be placed into the file without comment. See MPEP 2733.
See the example notification letter for details. When auditing PTA, patentees are reminded to check PTA vis-à-vis the "official" USPTO PTA determination as printed on the patent (in addition to the calculation details available in PAIR). If you have questions, please contact us via e-mail to Support@PatentTerm.com.
April 17, 2016: District Court Dismisses B-Delay PTA Challenge In Singhal v. Lee, 1:12-cv-00708 (E.D. Va. 2016), the US District Court for the Eastern District of Virginia dismissed a pro se challenge to the PTA statute defining B-Delay. B-Delay is a type of PTA under 35 USC 154(b)(1)(B) accruing if the USPTO fails to issue a patent within three years. B-Delay does not include inter alia "any time consumed by continued examination of the application requested by the applicant under section 132(b)." 35 USC 154(b)(1)(B)(i). Although seemingly contrary to 35 USC 154(b)(1)(B)(i), Singhal argued that time consumed by RCE's should not be excluded from B-Delay because the statute, in regards to defining the term "request for continued examination," is unconstitutionally vague. The Court squarely rejected this contention: Plaintiff's argument fails for at least two reasons: (1) the constitutional "void for vagueness" doctrine only applies to statutes that, unlike § 154, seek to prohibit conduct; and (2) § 154's use of "request for continued examination" especially when the provision includes a cross-reference to the statute creating the procedure itself is clearly comprehensible. Singhal further argued against RCE exclusions on policy grounds, including that Congress failed to comprehend that applicants often have good reason to file an RCE. The Court refused to consider such policy arguments: The remaining arguments simply take issue with the policy judgment that Congress made in promulgating § 154. . . . Courts including the Federal Circuit in interpreting § 154 itself have repeatedly held that they lack authority to re-write a statute simply because a Plaintiff "believes that the delicate balance that Congress struck was erroneous, unwise, or somehow inequitable." Wyeth v. Kappos, 591 F.3d 1364, 1370-71 (Fed. Cir. 2008). Plaintiff has not identified any authority to the contrary. This dismissal is consistent with how the USPTO, and most applicants, typically calculate PTA. The above overview is general in nature. Please see the District Court opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
January 26, 2016: USPTO Storm-Related Closing Affects PTA Due to weather conditions in the DC area, the USPTO was closed on Monday, January 25, 2016, and Tuesday, January 26, 2016. The USPTO will consider these days to be “Federal holiday[s] within the District of Columbia” under 35 USC § 21. Accordingly, where a 3-month 1.704(b) applicant response deadline falls on these dates (or on Saturday, January 23, 2016, or Sunday, January 24, 2016), the effective 3-month deadline for determining any PTA reduction is extended to Wednesday, January 27, 2016. See ArQule v. Kappos, 793 F.Supp.2d 214 (D.D.C. 2011); MPEP § 2732 (Ninth Edition, Revision 07.2015, Oct. 2015) at 2700-28. We have updated our calculator to automatically consider this closing in analyzing PTA rules when the Apply ArQule v. Kappos option is selected on the Apply Term Rules tab. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
January 22, 2016: Federal Circuit Upholds USPTO PTA Calculation [Updated] Today, in Pfizer v. Lee, 2015-1265 (Fed. Cir. 2016), a split panel of the US Court of Appeals for the Federal Circuit upheld the USPTO's determination that its restriction requirement, even though incomplete and subsequently withdrawn, constituted a notification under 35 USC § 132 for purposes of satisfying its 14-month first action PTA deadline under 35 USC § 154(b)(1)(A)(i). A-Delay is a type of PTA under 35 USC § 154(b)(1)(A) accruing if the USPTO fails to act by certain examination deadlines. The deadline at issue in Pfizer requires the USPTO to provide a Section 132 notification (e.g., a restriction requirement or office action), or a notice of allowance, within 14 months of the filing or national stage commencement date. See 35 USC § 154(b)(1)(A)(i). In the prosecution of the patent on appeal, the USPTO first provided an incomplete restriction requirement which omitted the classification of six dependent claims. Once informed of this deficiency by the Applicant, the Examiner acknowledged that the restriction was not complete, agreed to withdraw it, and issued a corrected restriction requirement. In its challenge to the USPTO-calculated PTA, Pfizer argued that the corrected restriction requirement, not the withdrawn one, ended the USPTO 14-month deadline, thus requiring a greater PTA. The Pfizer majority rejected this contention, holding inter alia: [T]he examiner’s initial restriction requirement satisfied the statutory notice requirement because it informed the applicant of “the broad statutory basis for [the rejection of] his claims.” Here, the examiner’s detailed descriptions of the 21 distinct invention groups outlined in the examiner’s initial restriction requirement were clear, providing sufficient information to which the applicants could have responded.… Indeed, the applicants never challenged the content of the invention groups defined by the examiner. And, significantly, the examiner’s defined invention groups remained identical between the two restriction requirements.… Viewed as a whole, the restriction requirement provided adequate grounds on which the applicants could “recogniz[e] and seek[] to counter the grounds for rejection.” Therefore, because the examiner clearly defined to the applicants the invention groups available for election and further prosecution, the applicants were placed on sufficient notice of the reasons for the examiner’s restriction requirement. * * * [T]he applicants’ and examiner’s exchanges concerning the challenged restriction requirement were part of the typical “back and forth” process of patent prosecution. The underlying “purpose of PTA is to ‘compensate patent applicants for certain reductions in patent term that are not the fault of the applicant,’ not to guarantee the correctness of the agency’s every decision.” As explained above, because the initial restriction requirement placed the applicants on notice of “the broad statutory basis for [the rejection of their] claims,” the restriction requirement satisfied the notice requirement of Section 132. Thus, we conclude that Appellants’ alleged delay is not the type of error for which the Act was intended to compensate. Slip Op. at 10-16 (citations omitted). The Court distinguished situations where an independent claim is omitted from a restriction requirement, where an examiner revises the nature or description of the distinct inventions, and where an examiner sua sponte rescinds and replaces a restriction without explanation or prompting from the applicant. While not dispositive, such circumstances may support an argument that certain withdrawn actions do not satisfy the notice requirement of Section 132. Judge Newman dissented, stating inter alia: Despite the PTO’s admission of its error, my colleagues propose that the applicant should have proceeded as if the incorrect restriction requirement were correct “because the initial restriction requirement placed the applicant on notice of ‘the broad statutory basis for [the rejection of] his claims,’ the restriction requirement satisfied the notice requirement of Section 132.” Thus the panel majority holds that this admitted PTO-caused delay must be treated as if it did not occur, although it necessarily delayed prosecution, for the applicant could not reliably elect which claims to prosecute when some claims had been omitted by the examiner. The issue is not whether the applicant could have guessed where among the 21 groups the examiner intended to put claims 75, 76, 103, 104, 105, and 106. Nor is the issue whether the error was harmless (the PTO does not argue that its error was harmless), for it is not disputed that the error delayed prosecution. The PTO does not argue that the prosecution could have proceeded in the absence of PTO correction of its failure to account for every claim. The issue is simply whether the delay that necessarily ensued is an “‘A’ Delay” subject to inclusion in the term adjustment. * * * Rather than guess, the applicant is entitled to a complete Office action. See 37 C.F.R. § 1.104(b). Here, the PTO provided an incomplete action. The delay caused by such a scenario should not be charged against the patent applicant, nor should the applicant be prejudiced by the examiner’s error. The panel majority erroneously holds that term adjustment is not available because the applicant, not the PTO, spotted the PTO’s error. Whether the examiner’s actions “were outside the normal ‘give-and-take process’ of patent prosecution,” should not turn on who recognized the error. * * * The statutory purpose is clear: when patent issuance is delayed because of proceedings that are not the fault of the applicant, the patent term is extended to compensate for the delay. My colleagues’ statutory interpretation and application are contrary to the letter and purpose of the law. I respectfully dissent. Slip Op. at 3-7 (citations omitted). Note that, in contrast to the PTA effect of USPTO conduct, where an applicant submits a reply having an omission, or supplements its reply (unless expressly requested by the Examiner), such actions will typically generate a PTA reduction. See 37 CFR 1.704(c)(7),(8). The above overview is general in nature. Please see the Federal Circuit opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
December 29, 2015: Emergency USPTO Closing Affects PTA Due to a major outage of many USPTO electronic systems, the USPTO will consider each day from Tuesday, December 22, 2015, to Thursday, December 24, 2015, to be a "Federal holiday within the District of Columbia" under 35 USC § 21. Accordingly, where a 3-month 1.704(b) applicant response deadline falls on one of these days, the effective 3-month deadline for determining any PTA reduction is extended to Monday, December 28, 2015. See ArQule v. Kappos, 793 F.Supp.2d 214 (D.D.C. 2011); MPEP § 2732 (Ninth Edition, Revision 07.2015, Oct. 2015) at 2700-28. Note that December 25, 26, and 27, 2015, are a Federal holiday, Saturday, and Sunday, respectively. We have updated our calculator to automatically consider this emergency closing in analyzing PTA rules when the Apply ArQule v. Kappos option is selected on the Apply Term Rules tab. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
November 14, 2015: USPTO Revises MPEP Chapter 2700 on Patent Terms and Extensions The USPTO recently issued MPEP, Ninth Edition, Revision 07.2015, including a revised Chapter 2700 on Patent Terms and Extensions. This version updates the MPEP's discussion of patent term adjustment (PTA) provisions in view of, and for consistency with, several recent court decisions and rule changes, including: - Mohsenzadeh v. Lee, 790 F.3d 1377 (Fed. Cir. 2015)
- Gilead Sciences, Inc. v. Lee, 778 F.3d 1341 (Fed. Cir. 2015)
- Novartis AG v. Lee, 740 F.3d 593 (Fed. Cir. 2014)
- Final Rule, Changes to Patent Term Adjustment in view of the Federal Circuit Decision in Novartis v. Lee, 80 FR 1346 (Jan. 9, 2015)
- Final Rule, Revisions To Implement the Patent Term Adjustment Provisions of the Leahy-Smith America Invents Act Technical Corrections Act, 79 FR 27755 (May 15, 2014)
- Final Rule, Changes to Implement the Patent Law Treaty, 78 FR 62368 (Oct. 21, 2013)
See below for more detailed information on the impact of each of these PTA developments. The above highlights are general in nature. Please see the revised Chapter 2700 for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
July 03, 2015: Federal Circuit Rejects Retroactive PTA Recalculation under Wyeth In Daiichi Sankyo Co. v. Lee, 2014-1280 (Fed. Cir. 2015), the US Court of Appeals for the Federal Circuit rejected Daiichi's attempt to retroactively apply the holding of Wyeth after expiration of the 180-day civil review deadline set forth in 35 USC 154(b)(4)(A) (2006) (amended 2011). As detailed in prior posts below, in Wyeth v. Kappos, 591 F.3d 1364 (Fed. Cir. 2010), the Federal Circuit held that the USPTO was under-calculating PTA for many patents. In response, the USPTO created an expedited Optional Interim Procedure (now expired) for patentees to request PTA reconsideration under Wyeth without filing a petition under 37 CFR 1.705(d) or a district court action under 35 USC 154(b)(4)(A) (2006). The USPTO limited use of the procedure, however, to patents still eligible for civil review of PTA under 35 USC 154(b)(4)(A) (2006). Nonetheless, under several different legal theories, Daiichi contended that two of its older patents, issued outside of the 180-day deadline of 35 USC 154(b)(4)(A) (2006), should have been entitled to PTA recalculation under Wyeth. The Federal Circuit rejected Daiichi's arguments. For example, in upholding the USPTO's time limitation to seek administrative review, the Court stated: Regarding the [USPTO's] adoption of the 180-day period for administrative review, the government argues that this selection was logical because it lengthened the period for administrative review to match the period for seeking judicial review. Additionally, the government points to the abbreviated period of judicial review as evidence of Congress' intent that questions regarding patent term adjustments be "decided quickly and soon after the issuance of the patent." Hence, any extension of the period for administrative review beyond 180 days would be contrary to the statute. Finally, the government contends that to consider any recalculation request regardless of how long after issuance it was filed--as Daiichi claims is appropriate under 35 USC § 254--would run contrary to the PTO’s authority to adopt regulations governing the procedures for requesting reconsideration of patent term adjustments. * * * Here, we agree with the government that the PTO has not erroneously interpreted the law. Rather, the agency acted within its discretion under the statute to “prescribe regulations establishing procedures for the . . . determination of patent term adjustments,” 35 USC § 154(b)(3), in adopting the 180-day period as part of the Interim Procedure. Slip Op. at 11 (citations omitted). Although not in effect in Daiichi, note that 35 USC 154(b)(4)(A) was substantially revised by HR 6621, signed into law on January 14, 2013. For patents in which the revised statute applies, before filing a civil action, an applicant must now arguably first request PTA reconsideration in the USPTO, and the 180-day deadline runs from the USPTO's decision on the request: An applicant dissatisfied with the Director’s decision on the applicant’s request for reconsideration under paragraph (3)(B)(ii) shall have exclusive remedy by a civil action against the Director filed in the United States District Court for the Eastern District of Virginia within 180 days after the date of the Director’s decision on the applicant’s request for reconsideration. Chapter 7 of title 5 shall apply to such action. Any final judgment resulting in a change to the period of adjustment of the patent term shall be served on the Director, and the Director shall thereafter alter the term of the patent to reflect such change. 35 USC 154(b)(4)(A) (2013). See below for more information on HR 6621. The above overview is general in nature. Please see the Federal Circuit opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
June 25, 2015: Federal Circuit Upholds PTA Calculation Today, in Mohsenzadeh v. Lee, 2014-1499 (Fed. Cir. 2015), the US Court of Appeals for the Federal Circuit upheld the USPTO's long-standing position that PTA accrued in a parent application does not carry over to a continuing application. Mohsenzadeh argued that a 1,476 day adjustment, based on a late restriction requirement in a parent application, should also carry over to two patents issued on divisional applications. The adjustment arose in the parent application because the USPTO failed to issue a first action within 14 months of the filing date as required under 35 USC § 154(b)(1)(A)(i). The Court rejected this argument: The language of the provision of the patent term adjustment statute at issue, 35 USC § 154(b)(1)(A), clearly shows that Congress intended delay in the prosecution of an application to be restored to a single patent, the patent issuing directly from that application. In other words, the term of any patent arising from a continuing application is not restored for delay in the prosecution of the parent patent’s application. Slip Op. at 9. Of course, a patent issuing from a continuing application is still entitled to its own PTA based on delays occurring in its own prosecution. This ruling is consistent with how the USPTO, and most applicants, typically calculate PTA. See the Federal Circuit opinion for complete details.
May 29, 2015: Crystal Anniversary of AIPA Patent Term Adjustment To restore fairness and proper incentives to inventors, the American Inventors Protection Act (AIPA) was enacted in 1999 to compensate for Patent Office examination delays. AIPA guaranteed that diligent applicants would always receive a patent term of no less than 17 years, sometimes considerably more, up to 20 years. Today marks the 15th anniversary of the applicability of AIPA Patent Term Adjustment. Original applications (other than for a design patent) are subject to AIPA Patent Term Adjustment if filed on or after May 29, 2000.
March 20, 2015: PTA on Agenda at Next USPTO BCP Customer Partnership Meeting The USPTO has scheduled its next Biotechnology, Chemical and Pharmaceutical Customer Partnership (BCP) Meeting for April 7, 2015. The agenda includes a session on recent updates in Patent Term Adjustment (PTA) and Patent Term Extension (PTE) by Mary Till and Kery Fries of the USPTO Office of Patent Legal Administration. The registration deadline is April 3, 2015. Participants can attend in person or via webcast. Visit the event page for registration and more information.
March 10, 2015: New PTA Reduction Goes into Effect A new PTA reduction, for filing an RCE after a Notice of Allowance, goes into effect today under 37 CFR 1.704(c)(12): Circumstances that constitute a failure of the applicant to engage in reasonable efforts to conclude processing or examination of an application also include ... (12) Submission of a request for continued examination under 35 USC 132(b) after any notice of allowance under 35 USC 151 has been mailed, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the day after the date of mailing of the notice of allowance under 35 USC 151 and ending on the date the request for continued examination under 35 USC 132(b) was filed; The amount of the reduction equals the number of days from the day after the mailing of the notice of allowance until the RCE filing date. For example, an RCE filed 45 days after the mailing of a notice of allowance, will generate a 45 day PTA reduction. Note that 37 CFR 1.704(d) provides a "safe harbor" exempting some RCE/IDS filings from this new PTA reduction. To qualify, the RCE must only submit a compliant IDS, and must be accompanied by a statement under Rule 1.704(d) that: [E]ach item of information contained in the information disclosure statement: (i) Was first cited in any communication from a patent office in a counterpart foreign or international application or from the Office, and this communication was not received by any individual designated in § 1.56(c) more than thirty days prior to the filing of the information disclosure statement; or (ii) Is a communication that was issued by a patent office in a counterpart foreign or international application or by the Office, and this communication was not received by any individual designated in § 1.56(c) more than thirty days prior to the filing of the information disclosure statement. See 37 CFR 1.704(d) for complete details. The new 37 CFR 1.704(c)(12) PTA reduction "applies only to applications in which a request for continued examination under 35 USC 132(b) and 37 CFR 1.114 is filed on or after March 10, 2015." 80 FR 1346 (Jan. 9, 2015). Based on this wording, arguably, the reduction may apply retroactively to older RCE's if a subsequent RCE is filed on or after March 10, 2015 in the same application. Most post-grant PTA audits will not immediately be impacted (until affected applications proceed to issuance). Practitioners should, however, consider the new PTA reduction and its safe harbor exemption in determining prosecution strategy. We have added a new rule in our calculator, RCE after Notice of Allowance, corresponding to the new 37 CFR 1.704(c)(12) PTA reduction. This rule can be added on the Apply Term Rules tab. The above description is general in nature, and the PTA effect in an individual patent will depend on the specifics of its prosecution. Please see the Final Rule for complete details. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
February 26, 2015: Federal Circuit Upholds PTA Reduction Today, in Gilead Sciences, Inc. v. Lee, 2014-1159 (Fed. Cir. 2015), the US Court of Appeals for the Federal Circuit affirmed summary judgment upholding the USPTO's PTA reduction for applicant delay pursuant to 37 CFR 1.704(c)(8). The specific conduct at issue was the applicant's filing of a supplemental IDS after a response to a restriction requirement had been filed. 35 USC 154(b)(2)(C)(i) mandates that PTA be reduced by a period equal to "time during which the applicant failed to engage in reasonable efforts to conclude prosecution of the application." 35 USC 154(b)(2)(C)(iii) requires the USPTO to "prescribe regulations establishing the circumstances that constitute a failure of an applicant to engage in reasonable efforts to conclude processing or examination of an application." Pursuant to this authority, the USPTO promulgated Rule 1.704(c)(8): Circumstances that constitute a failure of the applicant to engage in reasonable efforts to conclude processing or examination of an application also include ... (8) Submission of a supplemental reply or other paper, other than a supplemental reply or other paper expressly requested by the examiner, after a reply has been filed, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the day after the date the initial reply was filed and ending on the date that the supplemental reply or other such paper was filed; The Gilead applicant argued inter alia that its supplemental IDS, filed after a response to a restriction requirement, should not generate a PTA reduction because it did not cause any actual prosecution delay. The Federal Circuit rejected this argument stating: [T]his court finds that a reasonable interpretation of the statute is that Congress intended to sanction not only applicant conduct or behavior that result in actual delay, but also those having the potential to result in delay irrespective of whether such delay actually occurred. Slip Op. at 14. After considering this and several related arguments, the Court ultimately rejected Gilead’s contention that the regulation is overbroad and found the USPTO’s construction of the statute to be reasonable. Note that practitioners may sometimes, in appropriate circumstances, avoid a PTA reduction by filing an IDS concurrently with or before a reply to an Office action, or by including a statement under the safe harbor provision of 37 CFR 1.704(d). Further note that Rule 1.704(c)(8) is not limited to IDS filings, but also applies to certain other applicant papers such as a supplemental reply or petition. The above description is general in nature, and the PTA effect in an individual patent will depend on the specifics of its prosecution. Please see the Gilead opinion for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
January 15, 2015: Calculator Update We have updated our PTA calculator in accordance with the recent revision to 37 CFR 1.703(b)(1) set forth in Changes to Patent Term Adjustment in view of the Federal Circuit Decision in Novartis v. Lee, 80 FR 1346 (Jan. 9, 2015), which is effective immediately for all patents subject to AIPA patent term adjustment. See our prior post for more information on this Final Rule. This calculator update affects one rule in our system, Exclusion for Continued Examination. For this rule, we have updated its description, changed its default behavior to count the last day of the exclusion period as an exclusion day (changeable by user election), and changed the exclusion ending event title to "Notice of Allowance." See the Apply Term Rules tab to view the new version in your applications. Importantly, for existing applications, existing rule assignments are not automatically changed unless you rerun the Rule Assignment Engine by (i) clicking QuickStart on the Applications tab; or (ii) clicking Apply Draft Rules on the Apply Term Rules tab. Of course, as always, you must carefully review draft rule assignments and alter, add, or delete rules to perform the PTA calculation in accordance with your legal interpretations. In addition, we will be adding a new rule corresponding to the new PTA reduction in 37 CFR 1.704(c)(12) prior to its effective date of March 10, 2015. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
January 12, 2015: USPTO Issues Final Rules on Novartis [Updated] The USPTO has issued a Final Rule, Changes to Patent Term Adjustment in view of the Federal Circuit Decision in Novartis v. Lee, 80 FR 1346 (Jan. 9, 2015), setting forth final rule changes in light of Novartis AG v. Lee, 740 F.3d 593 (Fed. Cir. 2014). The Notice finalizes the USPTO's Proposed Rule of June 18, 2014, discussed below, implementing three primary changes: (I) Revises USPTO Interpretation of "Time Consumed by Continued Examination" in light of Novartis B-Delay is a type of PTA under 35 USC 154(b)(1)(B) accruing if the USPTO fails to issue a patent within three years. B-Delay does not include inter alia "any time consumed by continued examination of the application requested by the applicant under section 132(b)." 35 USC 154(b)(1)(B)(i). The Novartis Court partially rejected the USPTO's calculation of such excluded time, holding that “time consumed by continued examination, 35 USC § 154(b)(1)(B)(i), is time up to allowance, but not later, unless examination on the merits resumes." 740 F.3d at 602 (internal quotation marks omitted). To implement this holding, the USPTO has revised 37 CFR 1.703(b)(1) to exclude from B-Delay: The number of days, if any, in the period beginning on the date on which any request for continued examination of the application under 35 USC 132(b) was filed and ending on the date of mailing of the notice of allowance under 35 USC 151; This formulation, which simply excludes the period from each RCE to the next notice of allowance, is less complex than the Proposed Rule, and does not exclude periods where the USPTO reopens prosecution after allowance. The day of allowance, however, is excluded from B-Delay, which is arguably contrary to Novartis and will likely be the subject of future litigation (see discussion of Proposed Rule below and comments submitted in response to Proposed Rule). This rule change is effective immediately and applies, prospectively and retroactively, to all patents subject to AIPA patent term adjustment. According to the Notice, the USPTO's PTA-calculation algorithm has already been updated for PTA determinations in patents issued on or after October 7, 2014. Patentees are advised to recheck PTA in appropriate cases and seek correction as necessary. (II) Implements New PTA Reduction Wherein Filing an RCE after Allowance May Reduce PTA To ensure that applicants do not obtain additional B-Delay for the time after a notice of allowance by filing an RCE to delay issuance, the USPTO has implemented a new PTA reduction under 37 CFR 1.704(c): Circumstances that constitute a failure of the applicant to engage in reasonable efforts to conclude processing or examination of an application also include ... (12) Submission of a request for continued examination under 35 USC 132(b) after any notice of allowance under 35 USC 151 has been mailed, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the day after the date of mailing of the notice of allowance under 35 USC 151 and ending on the date the request for continued examination under 35 USC 132(b) was filed; The reduction provides no exception for cases in which there is no B-Delay, or less B-Delay than the reduction. Thus, based on its stated purpose, Rule 1.704(c)(12) will over penalize some patentees and, for a limited number of cases, provide a shorter patent term than was available prior to Novartis. It is possible some patentees will challenge the reduction, arguing that a post allowance RCE/IDS submission does not constitute a "failure of the applicant to engage in reasonable efforts to conclude processing or examination." Unlike the Proposed Rule, however, the USPTO has amended 37 CFR 1.704(d) to add a "safe harbor" exempting some post-allowance RCE/IDS filings from the new PTA reduction. To qualify, the RCE must only submit a compliant IDS, and must be accompanied by a statement under Rule 1.704(d) that: [E]ach item of information contained in the information disclosure statement: (i) Was first cited in any communication from a patent office in a counterpart foreign or international application or from the Office, and this communication was not received by any individual designated in § 1.56(c) more than thirty days prior to the filing of the information disclosure statement; or (ii) Is a communication that was issued by a patent office in a counterpart foreign or international application or by the Office, and this communication was not received by any individual designated in § 1.56(c) more than thirty days prior to the filing of the information disclosure statement. See amended 37 CFR 1.704(d) for complete details. The new 37 CFR 1.704(c)(12) PTA reduction applies only to applications in which an RCE is filed on or after March 10, 2015. (III) Clarifies Which "Other Papers," if Filed After Allowance, Reduce PTA 37 CFR 1.704(c)(10) provides for a PTA reduction if certain papers are filed by an applicant after allowance. The USPTO has revised this subsection to explicitly indicate that an RCE is not subject to the reduction: Circumstances that constitute a failure of the applicant to engage in reasonable efforts to conclude processing or examination of an application also include ... (10) Submission of an amendment under § 1.312 or other paper, other than a request for continued examination in compliance with § 1.114, after a notice of allowance has been given or mailed, in which case the period of adjustment set forth in § 1.703 shall be reduced by the lesser of: (i) The number of days, if any, beginning on the date the amendment under § 1.312 or other paper was filed and ending on the mailing date of the Office action or notice in response to the amendment under § 1.312 or such other paper; or (ii) Four months; (emphasis added). The revision to 37 CFR 1.704(c)(10) applies to all patents subject to AIPA patent term adjustment. Since the revision is consistent with current USPTO practice, however, no effect on PTA calculations is likely. Importantly, the Notice discussion also clarifies policies set forth in 1247 OG 111 (June 26, 2001) and MPEP 2732, providing information on some papers which are and are not subject to 37 CFR 1.704(c)(10). The Notice discussion states: [S]ubmission of the following papers after a notice of allowance will not be considered a failure to engage in reasonable efforts to conclude processing of examination of the application under § 1.704(c)(10): - (1) An Issue Fee(s) Transmittal (PTOL–85B);
- (2) a power of attorney;
- (3) a power to inspect;
- (4) a change of address;
- (5) a change of entity status (micro, small, non-small);
- (6) a response to the examiner’s reasons for allowance or a request to correct an error or omission in the ‘‘Notice of Allowance’’ or ‘‘Notice of Allowability’’;
- (7) status letters [policy change];
- (8) requests for a refund [policy change];
- (9) an inventor’s oath or declaration [policy change];
- (10) an information disclosure statement with a statement in compliance with § 1.704(d);
- (11) the resubmission by applicant of unlocatable paper(s) previously filed in the application (§ 1.251) [newly listed];
- (12) a request for acknowledgment of an information disclosure statement in compliance with §§ 1.97 and 1.98, provided that the applicant had requested that the examiner acknowledge the information disclosure statement prior to the notice of allowance, or the request for acknowledgement was applicant’s first opportunity to request that the examiner acknowledge the information disclosure statement [policy change];
- (13) comments on the substance of an interview where the applicant-initiated interview resulted in a notice of allowance [newly listed]; and
- (14) letters related to government interests (e.g., those between NASA and the Office).
* * * The Office no longer considers submission of a written (or other type of) status inquiry, request for refund, or an inventor’s oath or declaration to be a failure to engage in reasonable efforts to conclude processing and examination of the application under § 1.704(c)(10) due to the changes that have been brought about by the electronic filing and processing of patent applications. * * * An exemplary listing of such papers [generating a Rule 1.704(c)(10) PTA reduction] includes: - (1) An amendment under § 1.312;
- (2) a paper containing a claim for priority or benefit or request to correct priority or benefit information (e.g., a new or supplemental application data sheet filed to correct foreign or domestic benefit information) [revised];
- (3) a request for a corrected filing receipt; [newly listed];
- (4) a certified copy of a priority document;
- (5) drawings;
- (6) a letter relating to biologic deposits;
- (7) a request to change or correct inventorship [newly listed]; and
- (8) an information disclosure statement not accompanied by a statement in compliance with § 1.704(d).
80 FR at 1354 (emphasis, line breaks, and bullets added; some listed items may merely reflect current USPTO practice). Since the Notice discussion modifies whether some applicant papers are subject to 37 CFR 1.704(c)(10), it may immediately impact PTA calculations for a limited number of patents. For affected cases, patentees are advised to recheck PTA and seek correction as appropriate. The above description is general in nature, and the PTA effect in an individual patent will depend on the specifics of its prosecution. Please see the Final Rule for complete details. We are in the process of updating our calculator in accordance with the Final Rule. In the meantime, when analyzing cases, you will need to manually adjust PTA rules on the Apply Term Rules tab to account for changes. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
January 09, 2015: USPTO Issues Final Rules on Novartis Today, the USPTO issued a Final Rule, Changes to Patent Term Adjustment in view of the Federal Circuit Decision in Novartis v. Lee, 80 FR 1346 (Jan. 9, 2015), setting forth final rule changes in light of the Federal Circuit decision in Novartis AG v. Lee, 740 F.3d 593 (Fed. Cir. 2014). We are currently reviewing this Final Rule and will add a post to highlight its changes. Please check back. We will be updating our calculator in accordance the Final Rule. In the meantime, when analyzing cases, you will need to manually adjust PTA rules on the Apply Term Rules tab to account for this change. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
December 17, 2014: USPTO Processed Nearly 10,000 Patent Term Petitions in FY 2014 According to the recent FY 2014 USPTO Performance and Accountability Report, the USPTO issued actions on 9,957 Patent Term Adjustment/Extension petitions in FY 2014, a ten-fold increase from the previous year. Most actions, however, were likely computer-generated recalculations under the optional procedure (now expired) allowing some patentees to request PTA recalculation in view of the AIA Technical Corrections Act without a fee or Rule 1.705 petition. See below for more information on the optional procedure (now expired) and the AIA Technical Corrections Act.
October 08, 2014: USPTO Updates PTA Algorithm for Novartis For at least some patents issuing on or after October 7, 2014, we have observed that the USPTO has apparently changed its PTA calculation algorithm to consider the effect of Novartis AG v. Lee, 740 F.3d 593 (Fed. Cir. 2014). This USPTO update, however, seems premature since the USPTO has not yet issued final rules on Novartis, and its Notice of proposed rulemaking was the subject of considerable commentary on the proper Novartis interpretation. Until the USPTO issues final rules on Novartis and the USPTO algorithm change is fully scrutinized, patentees are advised to carefully recheck USPTO-calculated PTA in light of Novartis and seek correction in appropriate cases. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
September 09, 2014: Comments Published on Proposed Rules The USPTO has published comments submitted in response to the Notice of proposed rulemaking, Changes to Patent Term Adjustment in View of the Federal Circuit Decision in Novartis v. Lee, 79 FR 34681 (June 18, 2014). Commenters include AIPLA, IPO, and PhRMA. See below for more information on this important Notice of proposed rulemaking.
June 18, 2014: USPTO Issues Proposed Rules on Novartis [Updated] The USPTO has issued a Notice of proposed rulemaking, Changes to Patent Term Adjustment in View of the Federal Circuit Decision in Novartis v. Lee, 79 FR 34681 (June 18, 2014). The Notice sets forth several proposed rule changes in light of the recent Federal Circuit decision in Novartis AG v. Lee, 740 F.3d 593 (Fed. Cir. 2014). Written comments concerning the Notice must be received by the USPTO on or before August 18, 2014. Under 35 USC 154(b)(1)(B), if the USPTO fails to issue a patent within three years, a type of PTA--commonly known as B-Delay--begins to accrue. Per 35 USC 154(b)(1)(B)(i), B-Delay does not include inter alia "any time consumed by continued examination of the application requested by the applicant under section 132(b)." In Novartis, the Court held that the USPTO was overcalculating the amount of time consumed by continued examination, thus undercalculating B-Delay: [W]e agree with Novartis on its second § 154(b)(1)(B) issue. Novartis argues that the “time consumed by continued examination” should be limited to the time before allowance, as long as no later examination actually occurs. In contrast, the PTO contends that any time up until the patent issues, even after allowance, should be excluded from the adjustment awarded to the patentee. We reject the PTO’s view that the time after allowance, until issuance, is “time consumed by continued examination” and so is excluded from adjustments given to the patentee. Such time from allowance to issuance undisputedly would count toward the PTO’s three-year allotment in a case not involving a continued examination. There is no basis for distinguishing a continued examination case. * * * [A]llowance-to-issuance time is not to be distinguished according to whether there is a continued examination in a prosecution. Either way such time is plainly attributable to the PTO. The language of “examination” used in § 154(b)(1)(B) reflects that underlying principle. An “examination” presumptively ends at allowance, when prosecution is closed and there is no further examination on the merits in the absence of a special reopening.... The common-sense understanding of “time consumed by continued examination,” 35 USC § 154(b)(1)(B)(i), is time up to allowance, but not later, unless examination on the merits resumes. The PTO identifies several circumstances in which affirmative action is taken to resume examination after allowance, perhaps based on new information submitted by applicants in fulfillment of their continuing duty to disclose information material to patentability, 37 CFR § 1.56. See, e.g., BlackLight Power, Inc. v. Rogan, 295 F.3d 1269, 1273-74 (Fed. Cir. 2002) (even after payment of the issue fee, but before issuance, PTO officials can take “extraordinary action to withdraw a patent from issue” and “return the . . . application to examination”); 37 CFR § 1.313(a) (applicant may request resumption of examination). But such circumstances are exceptional, and an appropriate adjustment can be made when they occur. For none of the three applications at issue does the PTO identify any “continued examination of the application” that occurred after the notice of allowance was mailed. The possible existence of these exceptional cases does not support a general rule excluding time between allowance and issuance. In the present case, time after allowance was not time caused by the continued examination. Because the PTO applied the contrary view in calculating the patent term adjustment for the [patents at issue], those calculations must be corrected. 740 F.3d at 602. To implement the Novartis holding, in proposed Rule 1.703(b)(1), the USPTO is putting forth a somewhat complex formula demarcating time consumed by continued examination: The number of days, if any, in the period beginning on the date on which a request for continued examination of the application under 35 USC 132(b) was filed and ending on the date of mailing of a notice of allowance under 35 USC 151, unless prosecution in the application is reopened, in which case the period of adjustment under § 1.702(b) also does not include the number of days, if any, in the period or periods beginning on the date on which a request for continued examination of the application under 35 USC 132(b) was filed or the date of mailing of an action under 35 USC 132, whichever occurs first, and ending on the date of mailing of a subsequent notice of allowance under 35 USC 151; Accordingly, where prosecution in an application is reopened after allowance, an RCE would generate non-contiguous periods excluded from B-Delay (e.g., the period from an RCE to a first allowance, and the period from a subsequent Office action reopening prosecution to the next allowance). Moreover, regardless of whether prosecution is reopened, the formula excludes the day of allowance, which is arguably contrary to Novartis ("[A]llowance-to-issuance time is not to be distinguished according to whether there is a continued examination in a prosecution.... The common-sense understanding of “time consumed by continued examination ... is time up to allowance, but not later, unless examination on the merits resumes.") (emphasis added). In addition, to penalize applicants for potentially obtaining multiple periods of B-Delay by filing an RCE after a notice of allowance, the USPTO is proposing a new PTA reduction under Rule 1.704(c): Circumstances that constitute a failure of the applicant to engage in reasonable efforts to conclude processing or examination of an application also include ... (12) Submission of a request for continued examination under 35 USC 132(b) after a notice of allowance under 35 USC 151 has been mailed, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the date of mailing of the notice of allowance under 35 USC 151 and ending on the date the request for continued examination under 35 USC 132(b) was filed; The reduction provides no exception for cases in which there is no B-Delay--patentees would still be subject to a PTA reduction. The Notice does not state whether or not the new penalty would apply retroactively. The Notice indicates that the USPTO has not yet modified its PTA calculation algorithm for Novartis. Thus, patentees are advised to recheck USPTO-calculated PTA and seek correction in appropriate cases. In deciding Rule 1.705 requests for PTA reconsideration, the USPTO manually calculates PTA and thus does take into account the Novartis decision. The above description is general in nature, and the potential PTA effect in an individual patent will depend on the specifics of its prosecution and the final implementation of the proposed changes. Please see the Notice of proposed rulemaking for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
May 16, 2014: USPTO Adopts Interim Final Rule The USPTO has issued a Final Rule, Revisions To Implement the Patent Term Adjustment Provisions of the Leahy-Smith America Invents Act Technical Corrections Act, 79 FR 27755 (May 15, 2014), adopting as final the rule changes of the Interim Final Rule of April 1, 2013, including: (1) For patents granted on or after January 14, 2013 on US national stage applications under 35 USC 371, the USPTO 14-month first action requirement under 35 USC 154(b)(1)(A)(i)(II) was changed. Under revised Rules 1.702(a)(1) and 1.703(a)(1), the USPTO must issue a first action within 14 months of commencement of the national stage under section 371(b) or (f), rather than upon fulfillment of the 371 requirements. Since the USPTO's 14-month deadline starts earlier in some national stage applications, this change is beneficial to patentees and may substantially increase PTA in some national stage cases. (2) For all patents granted on or after January 14, 2013, the PTA indicated on the patent itself is the USPTO's "official" notification of its PTA determination, and is the only determination for which an applicant may seek reconsideration under revised Rule 1.705. The deadline to seek such reconsideration is two months from the issue date, extendable by an additional five months pursuant to Rule 1.136(a). The USPTO will discontinue providing an indication of the PTA with the notice of allowance. (3) If an applicant needs to seek reinstatement of PTA where, in spite of all due care, it was unable to respond within 3 months (e.g., more time is necessary to obtain test data for an affidavit or declaration), the request must be filed prior to issuance. See 35 USC 154(b)(3)(C); 37 CFR 1.705(c). Such reinstatement request is not common, however. For USPTO calculation errors with respect to a three-month applicant response deadline, the notice clarifies that requesting reconsideration is by way of a Rule 1.705(b) petition. See 79 FR at 27759. Although there are no other rule changes beyond adopting those in the Interim Final Rule, the Final Rule commentary provides several important announcements concerning PTA correction: (4) According to the notice, the USPTO software performing PTA calculations has finally been modified, and PTA calculations for patents issuing on national stage applications on or after May 20, 2014 will finally take into account the revision per item (1) above. (5) Due to the significant delay in updating its software, the USPTO is providing an optional procedure for patentees to request PTA recalculation without a fee as an alternative to Rule 1.705(b). The optional procedure is only available for patents issuing between January 14, 2013 and May 20, 2014 directly from international applications. The request must be filed no later than July 31, 2014, and a request form PTO/SB/132 is available on the USPTO website. For purposes of the optional procedure, the USPTO has sua sponte waived the Rule 1.705(b) deadline and fee. Thus, even for some patents where the reconsideration request deadline has already passed, correction under the optional procedure may still be available. (6) The USPTO has detailed some new procedures concerning its treatment of requests for PTA reconsideration. As before, the USPTO will conduct a manual redetermination of PTA in response to a request for reconsideration, which could result in a PTA that is (i) the amount requested by the applicant, (ii) the same amount as indicated in the patent (i.e., no change), or (iii) a different amount that may be higher or lower than the PTA as indicated in the patent. In the latter case (or where more information is needed), the redetermination may not be considered the Director’s decision on the applicant’s request for reconsideration within the meaning of 35 USC 154(b)(4), and the patentee can and must seek further PTA reconsideration within the USPTO before filing a civil action under 35 USC 154(b)(4)(A): - If the PTA redetermination results in the PTA requested by the applicant, the USPTO will issue a decision granting the request for reconsideration and a certificate of correction indicating the revised PTA. If the PTA redetermination results in the same PTA as indicated in the patent (i.e., there being no change) and the USPTO does not require any additional information to render a decision on the request for reconsideration, the USPTO will issue a decision denying the request for reconsideration, and this decision is the Director’s decision on the applicant’s request for reconsideration within the meaning of 35 USC 154(b)(4).
- If the PTA redetermination results in a different PTA (higher or lower than the PTA indicated in the patent), the USPTO will issue a redetermination of PTA explaining how the USPTO arrived at the different PTA. This redetermination of PTA is not the Director’s decision on the applicant’s request for reconsideration within the meaning of 35 USC 154(b)(4), but is simply a new PTA determination. If the USPTO issues such a redetermination of PTA in response to the request for reconsideration of the PTA, the applicant has two months from the date of the redetermination of PTA to file a renewed request for reconsideration of the PTA (no additional fee required) that addresses the issues included in the USPTO’s redetermination of PTA. This two-month period is extendable under § 1.136(a).
- If the PTA redetermination results in the same PTA as indicated in the patent (i.e., there being no change) but the USPTO requires additional information to render a decision on the request for reconsideration of the PTA, the USPTO will issue a requirement for information to obtain the additional information. This requirement for information is not the Director’s decision on the applicant’s request for reconsideration within the meaning of 35 USC 154(b)(4). If the USPTO issues a requirement for information in response to the request for reconsideration of the PTA, the applicant has two months from the date of the requirement for information to file a renewed request for reconsideration of the PTA supplying the required information (no additional fee required). This two-month period is extendable under § 1.136(a).
- The USPTO will again conduct a redetermination of PTA in response to any renewed request for reconsideration in response to a redetermination of PTA or in response to a requirement for information. If this redetermination of PTA results in the PTA requested by the applicant, the USPTO will issue a decision granting the request for reconsideration and a certificate of correction indicating the revised PTA. If this redetermination of PTA results in the same PTA as indicated in the previous redetermination of PTA or in the patent, the USPTO will issue a decision denying the request for reconsideration (unless it is necessary to issue another redetermination of PTA or requirement for information) and a certificate of correction if necessary indicating the revised PTA as the result of a redetermination of PTA, and the decision denying the request for reconsideration is the Director’s decision on the applicant’s request for reconsideration within the meaning of 35 USC 154(b)(4).
(7) The USPTO reiterated its view that, under section 1(h) of the AIA Technical Corrections Act, a patentee must first seek PTA reconsideration in the USPTO under 35 USC 154(b)(3) prior to filing a civil action under 35 USC 154(b)(4)(A). See 79 FR at 27756 ("(1) A civil action under 35 USC 154(b)(4) is not an alternative to requesting reconsideration of a patent term adjustment under 35 USC 154(b)(3), but instead is the remedy for an applicant who is dissatisfied with the Director’s decision on the applicant’s request for reconsideration; and (2) a civil action under 35 USC 154(b)(4) is the exclusive remedy for an applicant who is dissatisfied with the Director’s decision on the applicant’s request for reconsideration."). (8) The USPTO has not yet modified its PTA calculation software per Novartis AG v. Lee, 740 F.3d 593 (Fed. Cir. 2014) (exclusion of RCE's from B-Delay), but is now beginning to manually process pending requests for reconsideration of PTA under Novartis. The USPTO is not adopting a special ad hoc procedure for patentees to request Novartis correction. Thus, patentees should seek PTA reconsideration per Rule 1.705(b) within two months from the issue date, extendable by an additional five months pursuant to Rule 1.136(a). The above description is general in nature, and the PTA effect in an individual patent will depend on the specifics of its prosecution. Please see the Final Rule for complete details. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
April 09, 2014: USPTO Revises MPEP Chapter 2700 on Patent Terms and Extensions The recently released MPEP edition, E9R-11.2013 (March 2014), includes a substantially revised Chapter 2700 on Patent Terms and Extensions. Most AIPA PTA-related revisions, however, are just reiterations of the numerous statutory and regulatory developments since the chapter's last update in May 2004. The chapter now incorporates the Wyeth holding (USPTO delays overlap only if occurring on the same calendar day) and the ArQule decision (3-month 1.704(b) applicant response deadline is extended for weekends and federal holidays within the District of Columbia), but it does not reference the recent Novartis decision (exclusion of RCE's from B-Delay). The revision does provide some clarification concerning Duty of Candor and Good Faith Letters, the treatment of which was the subject of a Federal Register Notice in July 2010. Revised MPEP 2733 provides in part: If a registered practitioner receives a patent term adjustment indicated on the front of the patent that is longer than expected, the practitioner may disclose the error to the Office in a letter in compliance with the practitioner’s duty of candor and good faith in practice before the Office. The Office will treat letters submitted by patentees stating that Office’s determination of patent term adjustment indicated on the patent is greater than what the applicant or patentee believes is appropriate by placing these letters in the file of the patent without comment. The Office will not review these letters or issue certificates of correction under either 35 USC 254 or 255 on the of these letters. In addition, the Office will not grant a request for a certificate of correction under either 35 USC 254 or 255 to revise the patent term adjustment indicated in a patent, unless the certificate of correction is issued to revised the patent for consistency with (1) the patent term adjustment determined via a decision on the request for reconsideration under 37 CFR 1.705; or (2) the total patent term adjustment indicated on the Patent Application Information Retrieval (PAIR) screen that displays the patent term adjustment calculation for the patent. If patentee submits a request for a certificate of correction under either 35 USC 254 or 255 to revise the patent term adjustment indicated in a patent that also includes changes in the patent for which a certificate of correction would be appropriate, the request for a certificate of correction will not be granted unless the patentee submits a new request for a certificate of correction that does not also attempt to revise the patent term adjustment indicated in the patent. If patentee wants the Office to reconsider its patent term adjustment determination, the patentee must use the procedures set forth in 37 CFR 1.705 for requesting reconsideration of a patent term adjustment determination. Specifically, the procedures set forth in 37 CFR 1.705 must be used whether the USPTO’s patent term adjustment determination is greater than or less than the adjustment that the applicant or patentee believes to be appropriate. A patentee may also file a terminal disclaimer at anytime disclaiming any period considered in excess of the appropriate patent term adjustment. See 35 USC 253 and 37 CFR 1.321. Note that the Office does not require patentee to file either a request for reconsideration under 37 CFR 1.705 or a terminal disclaimer when the patent term adjustment indicated on the patent is greater than what the patentee believes is appropriate. As discussed above, the patentee or the appointed registered practitioner may disclose the alleged error to the Office in a letter in compliance with the practitioner’s duty of candor and good faith. (emphasis added). This language provides a clearer indication of a USPTO view that (1) a Duty of Candor and Good Faith Letter is an acceptable disclosure; and (2) the disclosure letter may be filed after the patent issues (since it references the PTA indicated on the patent). Nonetheless, if a patentee wants the USPTO to actually reconsider its PTA determination on the merits and make a correction, rather than just place the disclosure letter in the file without comment, the patentee must seek reconsideration under Rule 1.705. For patents granted on or after January 14, 2013, MPEP 2733 reiterates that the patentee should seek PTA correction after issuance: [T]he patent term adjustment indicated on the patent is the“official” notification of the Office’s patent term adjustment determination under 35 USC 154(b). Accordingly, patentee should wait until the grant of the patent to determine whether or not a request for reconsideration of the patent term adjustment indicated on the patent is warranted. (emphasis added). Note, however, one uncommon exception. If an applicant needs to seek reinstatement of PTA where, in spite of all due care, it was unable to respond within 3 months (e.g., more time is necessary to obtain test data for an affidavit or declaration), the request must be filed prior to issuance. See 35 USC 154(b)(3)(C); 37 CFR 1.705(c). The above highlights are general in nature. Please see the USPTO's summary of changes and revised Chapter 2700 for complete details. If you have questions, please contact us via e-mail to Support@PatentTerm.com.
March 28, 2014: The USPTO has released a new MPEP edition, E9R-11.2013 (March 2014), including a substantially revised Chapter 2700 on Patent Terms and Extensions. The USPTO's summary of changes to Chapter 2700 is available here. We are currently reviewing revised Chapter 2700 and will provide additional information on this page. Please check back. In the meantime, if you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
March 27, 2014: In Mohsenzadeh v. Lee, 1:13-cv-00824 (E.D. Va. 2014), the US District Court for the Eastern District of Virginia upheld the USPTO's long-standing position that PTA accrued in a parent application does not carry over to a continuing application (at issue here, a divisional). The Court held: [T]he PTA Statute unambiguously applies to only one patent application--providing PTA only for delays that occurred during the prosecution of the application from which the patent issued. Alternatively, the Court holds that to the extent that the language of section 154(b)(1)(A) is ambiguous, the USPTO's longstanding interpretation of the statute, as manifested in 37 CFR §§ 1.702, 1.703, and 1.704(c)(12), is reasonable and entitled to some deference. Slip Op. at 24. Of course, a continuing application is still entitled to its own PTA based on events occurring during its own examination. See Id. at 23. This ruling is consistent with how the USPTO, and most applicants, typically calculate PTA. See the district court opinion for complete details.
January 23, 2014: We have updated our calculator to allow users to readily apply the Novartis holding (see case description below) in PTA calculations. Please logon to your account for details. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
January 15, 2014: [Updated] There has been a significant development in PTA law. Today, in Novartis AG v. Lee, 2013-1160 (Fed. Cir. 2014), the US Court of Appeals for the Federal Circuit partially adopted and partially rejected the USPTO's long-standing interpretation of how a Request for Continued Examination (RCE) affects the USPTO's obligation to issue a patent within three years under 35 USC 154(b)(1)(B) (commonly known as B-Delay). The ruling resolves conflicts between district court decisions in Novartis, Exelixis I, Exelixis II, and Abraxis. Agreeing with the USPTO, the Court held that an RCE, even if filed after the 3-year anniversary of the filing of a patent application, is excluded from USPTO B-Delay under 35 USC 154(b)(1)(B): [PTA] time should be calculated by determining the length of the time between application and patent issuance, then subtracting any continued examination time (and other time identified in (i), (ii), and (iii) of (b)(1)(B)) and determining the extent to which the result exceeds three years. Such a reading ensures that applicants recover for any “delay[s] due to the failure of the [PTO]," without allowing the applicant to recover for "any time consumed by continued examination," as the statute requires. Id. § 154(b)(1)(B)(i). * * * [T]ime spent in a continued examination does not deplete the PTO’s allotment of three years for application processing before a resulting patent has its term extended, no matter when the continued examination begins. Slip Op. at 14 (emphasis added). Thus, under Novartis, an applicant cannot avoid a B-Delay exclusion by merely waiting more than three years to file an RCE. Concerning the amount of time excluded from B-Delay, however, the Court rejected the USPTO's long-standing position, finding that a shorter exclusion is appropriate: [T]he PTO contends that any time up until the patent issues, even after allowance, should be excluded from the adjustment awarded to the patentee. We reject the PTO’s view that the time after allowance, until issuance, is “time consumed by continued examination” and so is excluded from adjustments given to the patentee. * * * The language of "examination" used in § 154(b)(1)(B) ... presumptively ends at allowance, when prosecution is closed and there is no further examination on the merits in the absence of a special reopening.... The common-sense understanding of "time consumed by continued examination," 35 USC § 154(b)(1)(B)(i), is time up to allowance, but not later, unless examination on the merits resumes. Slip Op. at 15. Another aspect of the Novartis decision concerns the 180-day deadline to file a civil action seeking PTA correction per 35 USC 154(b)(4) (2011 version). For a number of patents, Novartis attempted to avoid the deadline arguing, inter alia, (i) the deadline was inapplicable, (ii) the deadline was equitably tolled, and (iii) correction was available under the Takings Clause of the Fifth Amendment. The Court rejected each argument, but acknowledged one exception stating "[i]t is undisputed in this court that the 180-day clock does not run while the PTO considers a timely request for reconsideration under regulations promulgated pursuant to § (b)(3)(B)(ii)'s guarantee of "one opportunity to request reconsideration of any patent term adjustment determination made by the Director."" Slip Op. at 9. Although not in effect in Novartis, note that 35 USC 154(b)(4)(A) was substantially revised in January 2013. For patents for which the revised statute applies, before filing a civil action, an applicant must arguably first request PTA reconsideration in the USPTO, and the 180-day deadline runs from the USPTO's decision on the request. The Novartis decision will increase PTA for tens of thousands of issuing patents where an RCE was filed during prosecution, since PTA for USPTO B-Delay is now potentially available for the period after allowance (on average about four months). Be aware that the current USPTO PTA algorithm incorrectly extends the RCE exclusion until issuance. See 37 CFR 1.703(b)(1). To benefit from Novartis, applicants are advised to recheck PTA in patents where (i) the application was pending at least three years; and (ii) an RCE was filed (whether the RCE was filed before or after three years does not matter). Under the current version of 37 CFR 1.705, the deadline to seek PTA reconsideration in the USPTO is two months from the issue date, extendable by an additional five months pursuant to Rule 1.136(a). Under the current version of 35 USC 154(b)(4)(A), the deadline to file a civil action is within 180 days after the date of the Director's decision on the applicant's request for reconsideration. The above description is general in nature, and the PTA effect in an individual patent will depend on the specifics of its prosecution. Please see the opinion and legal authorities for complete details. We are working on updates to easily apply the Novartis holding in PTA calculations using our calculator. In the meantime, if you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
December 18, 2013: A new PTA reduction goes into effect today, wherein failing to provide an application in "condition for examination" within eight months from the filing or national stage commencement date constitutes applicant delay under 37 CFR 1.704(c)(12). See 78 FR 62368 (Oct. 21, 2013) and our post below for details. The new reduction applies only to patent applications filed under 35 USC 111 on or after December 18, 2013, and international patent applications in which the national stage commenced under 35 USC 371 on or after December 18, 2013. We have added a corresponding rule to our PTA calculator allowing users to perform a calculation in accordance with this new requirement. If you need assistance or have questions about how to apply the new rule, please contact us via e-mail to Support@PatentTerm.com.
November 05, 2013: [Exelixis Update] Today, Judges Newman, Dyk, and Taranto of the US Court of Appeals for the Federal Circuit heard oral argument on the following consolidated appeals: - Exelixis, Inc. v. Rea, No. 2013-1175, Appeal from the US District Court for the Eastern District of Virginia in Case No. 12-CV-0096, Judge T. S. Ellis, III ("Exelixis I")
- Exelixis, Inc. v. Rea, No. 2013-1198, Appeal from the US District Court for the Eastern District of Virginia in Case No. 12-CV-0574, Judge Leonie M. Brinkema ("Exelixis II")
- Novartis AG v. Rea, No. 2013-1160, -1179, Appeal from the US District Court for the District of Columbia in Consolidated Case No. 10-CV-1138, Judge Ellen S. Huvelle
These cases address, inter alia, a common substantive legal issue, the PTA effect of the filing of a Request for Continued Examination after the three-year anniversary of the filing of a patent application. See below for more information on this important legal issue. The timeliness of seeking judicial review of USPTO-calculated PTA is also at issue for some of the patents involved. A recording of the oral argument is available on the Federal Circuit website. We estimate a ruling will be issued in the first quarter of 2014.
October 22, 2013: [Updated] As part of the Final Rule, Changes to Implement the Patent Law Treaty, 78 FR 62368 (Oct. 21, 2013), the USPTO has promulgated a new PTA reduction wherein failing to provide an application in "condition for examination" within eight months from the filing or national stage commencement date will constitute applicant delay under 37 CFR 1.704(c): (12) Failure to provide an application in condition for examination as defined in paragraph (f) of this section within eight months from either - the date on which the application was filed under 35 U.S.C. 111(a) or
- the date of commencement of the national stage under 35 U.S.C. 371(b) or (f) in an international application,
in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the day after the date that is eight months from either - the date on which the application was filed under 35 U.S.C. 111(a) or
- the date of commencement of the national stage under 35 U.S.C. 371(b) or (f) in an international application
and ending on the date the application is in condition for examination as defined in paragraph (f) of this section; and * * * (f) An application filed under 35 U.S.C. 111(a) is in condition for examination when the application - includes a specification, including at least one claim and an abstract (§ 1.72(b)), and has
- papers in compliance with § 1.52,
- drawings (if any) in compliance with § 1.84,
- any English translation required by § 1.52(d) or § 1.57(a),
- a sequence listing in compliance with § 1.821 through § 1.825 (if applicable),
- the inventor’s oath or declaration or an application data sheet containing the information specified in § 1.63(b),
- the basic filing fee (§ 1.16(a) or § 1.16(c)),
- the search fee (§ 1.16(k) or § 1.16(m)),
- the examination fee (§ 1.16(o) or § 1.16(q)),
- any certified copy of the previously filed application required by § 1.57(a), and
- any application size fee required by the Office under § 1.16(s).
An international application is in condition for examination when - the application has entered the national stage as defined in § 1.491(b), and
- includes a specification, including at least one claim and an abstract (§ 1.72(b)), and has
- papers in compliance with § 1.52,
- drawings (if any) in compliance with § 1.84,
- a sequence listing in compliance with § 1.821 through § 1.825 (if applicable),
- the inventor’s oath or declaration or an application data sheet containing the information specified in § 1.63(b),
- the search fee (§ 1.492(b)),
- the examination fee (§ 1.492(c)), and
- any application size fee required by the Office under § 1.492(j).
An application shall be considered as having papers in compliance with § 1.52, drawings (if any) in compliance with § 1.84, and a sequence listing in compliance with § 1.821 through § 1.825 (if applicable) for purposes of this paragraph on the filing date of the latest reply (if any) correcting the papers, drawings, or sequence listing that is prior to the date of mailing of either an action under 35 U.S.C. 132 or a notice of allowance under 35 U.S.C. 151, whichever occurs first. 78 FR at 62408 (line breaks and bullets added). In response to comments on the proposed rule, the Final Rule clarifies that, apparently, corrections to application papers, drawings, and sequence listings after a first Office action (or notice of allowance) will not generate a PTA reduction under the new rule. See 78 FR at 62390 ("Thus, the patent term adjustment reduction provision of § 1.704(c)(12) would not apply to a correction of the application papers, drawings, or sequence listing that is required by an examiner (i.e., would not apply to corrections that take place after the date of mailing of either an action under 35 U.S.C. 132 or a notice of allowance under 35 U.S.C. 151)."). Note, however, that the USPTO commentary is somewhat different than the actual language of paragraph 1.704(f) ("An application shall be considered as having papers in compliance with § 1.52, drawings (if any) in compliance with § 1.84, and a sequence listing in compliance with § 1.821 through § 1.825 (if applicable) for purposes of this paragraph on the filing date of the latest reply (if any) correcting the papers, drawings, or sequence listing that is prior to the date of mailing of either an action under 35 U.S.C. 132 or a notice of allowance under 35 U.S.C. 151, whichever occurs first."). Accordingly, in some circumstances, it is possible there will be uncertainty on this point. The effective date of the Final Rule is December 18, 2013. The new PTA reduction under 37 CFR 1.704 applies only to patent applications filed under 35 USC 111 on or after December 18, 2013, and international patent applications in which the national stage commenced under 35 USC 371 on or after December 18, 2013. See the Final Rule for complete details. We are currently reviewing the Final Rule and will add a corresponding rule to our PTA calculator prior to the effective date. Please check back. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
October 22, 2013: In Actelion Pharma. Ltd. V. Kappos, No. 10-1145 (D.D.C. 2013), the US District Court for the District of Columbia rejected Actelion's attempt to retroactively apply the holding of Wyeth v. Kappos beyond the 180-day deadline set forth in 35 USC 154(b)(4)(A). In this action, a prior version of 154(b)(4)(A) applied: An applicant dissatisfied with a determination made by the Director under paragraph (3) [Procedures for patent term adjustment determination] shall have remedy by a civil action against the Director filed in the United States District Court for the District of Columbia within 180 days after the grant of the patent. Chapter 7 of title 5 [5 U.S.C. §§ 701-706] shall apply to such action ... 35 USC 154(b)(4)(A) (2010). In support of its claim, Actelion set forth several legal theories including: - 154(b)(4)(A) is a non-jurisdictional "claim-processing rule" subject to equitable tolling of the 180-day deadline;
- the USPTO's implementation of its post-Wyeth methodology and failure to provide a remedy of recalculating the applicant's PTA under that methodology violated the Administrative Procedure Act; and
- the USPTO's failure to correct the applicant's PTA was a "taking" of property without due process or just compensation in violation of the Fifth Amendment of the Constitution.
The Court rejected each theory and held that the 180-day time limit for seeking judicial review is in fact jurisdictional. Thus, because the applicant filed suit after the deadline, its statutory claim under 154(b)(4)(A) was untimely and the Court lacked subject matter jurisdiction to hear the claim. The Court also noted that general tolling, as applied in Bristol-Myers Squibb Co. v. Kappos, 841 F.Supp.2d 238 (D.D.C. 2012), did not apply in this case since the applicant never sought administrative reconsideration of the PTA. See the district court opinion for complete details. Although not in effect in Actelion, note that 35 USC 154(b)(4)(A) was substantially revised by HR 6621, signed into law on January 14, 2013. For patents in which the revised statute applies, before filing a civil action, an applicant must arguably first request PTA reconsideration in the USPTO, and the 180-day deadline runs from the USPTO's decision on the request: An applicant dissatisfied with the Director’s decision on the applicant’s request for reconsideration under paragraph (3)(B)(ii) shall have exclusive remedy by a civil action against the Director filed in the United States District Court for the Eastern District of Virginia within 180 days after the date of the Director’s decision on the applicant’s request for reconsideration. Chapter 7 of title 5 shall apply to such action. Any final judgment resulting in a change to the period of adjustment of the patent term shall be served on the Director, and the Director shall thereafter alter the term of the patent to reflect such change. 35 USC 154(b)(4)(A) (2013). See below for more information on HR 6621.
October 09, 2013: In Gilead Sciences, Inc. v. Rea, 1:12-cv-1090 (E.D. Va. 2013), the US District Court for the Eastern District of Virginia upheld the USPTO's long-standing position that an information disclosure statement, filed after a reply to a restriction requirement, and without a safe harbor statement under 37 CFR 1.704(d), constitutes applicant delay generating a PTA reduction under 37 CFR 1.704(c)(8) ("supplemental reply or other paper"). This ruling is consistent with how the USPTO, and most applicants, typically calculate PTA. See the district court opinion for complete details.
September 24, 2013: [Exelixis Update] The US Court of Appeals for the Federal Circuit has scheduled, for November 5, 2013, a consolidated oral argument before the same merits panel on the following cases: - Exelixis, Inc. v. Rea, No. 2013-1175, Appeal from the US District Court for the Eastern District of Virginia in Case No. 12-CV-0096, Judge T. S. Ellis, III ("Exelixis I")
- Exelixis, Inc. v. Rea, No. 2013-1198, Appeal from the US District Court for the Eastern District of Virginia in Case No. 12-CV-0574, Judge Leonie M. Brinkema ("Exelixis II")
- Novartis AG v. Rea, No. 2013-1160, -1179, Appeal from the US District Court for the District of Columbia in Consolidated Case No. 10-CV-1138, Judge Ellen S. Huvelle
These cases address, inter alia, a common substantive legal issue, the PTA effect of the filing of a Request for Continued Examination after the three-year anniversary of the filing of a patent application. See below for more information on this important legal issue.
April 14, 2013: As part of a Notice of Proposed Rulemaking, the USPTO has proposed a new PTA reduction wherein failing to provide an application in condition for examination within eight months of filing or national stage commencement will constitute applicant delay under 37 CFR 1.704(c): (11) Failure to provide an application in condition for examination as defined in paragraph (f) of this section within eight months from either the date on which the application was filed under 35 U.S.C. 111(a) or the date of commencement of the national stage under 35 U.S.C. 371(b) or (f) in an international application, in which case the period of adjustment set forth in § 1.703 shall be reduced by the number of days, if any, beginning on the day after the date that is eight months from either the date on which the application was filed under 35 U.S.C. 111(a) or the date of commencement of the national stage under 35 U.S.C. 371(b) or (f) in an international application and ending on the date the application is in condition for examination as defined in paragraph (f) of this section. * * * (f) An application filed under 35 U.S.C. 111(a) is in condition for examination when the application includes a specification, including at least one claim and an abstract (§ 1.72(b)), and has papers in compliance with § 1.52, drawings (if any) in compliance with § 1.84, any English translation required by § 1.52(d) or § 1.57(a), a sequence listing in compliance with § 1.821 through § 1.825 (if applicable), the inventor’s oath or declaration or application data sheet containing the information specified in § 1.63(b), the basic filing fee (§ 1.16(a) or § 1.16(c)), any certified copy of the previously filed application required by § 1.57(a), and any application size fee required by the Office under § 1.16(s). An international application is in condition for examination when the application has entered the national stage as defined in § 1.491(b), and includes a specification, including at least one claim and an abstract (§ 1.72(b)), and has papers in compliance with § 1.52, drawings (if any) in compliance with § 1.84, a sequence listing in compliance with § 1.821 through § 1.825 (if applicable), the inventor’s oath or declaration or application data sheet containing the information specified in § 1.63(b), and any application size fee required by the Office under § 1.492(j). Written comments concerning this proposed change must be received by June 10, 2013. See Changes to Implement the Patent Law Treaty, 78 FR 21788 (Apr. 11, 2013) for details.
April 03, 2013: [Updated] The USPTO has issued an Interim Final Rule implementing changes to the PTA provisions in section 1(h) of the AIA Technical Corrections Act, signed into law on January 14, 2013 (see our prior post below for an overview of these provisions). Highlights of the USPTO's Interim Final Rule include: (1) For patents granted on or after January 14, 2013 on US national stage applications under 35 USC 371, the USPTO 14-month first action requirement under 35 USC 154(b)(1)(A)(i)(II) has been changed. Under revised Rules 1.702(a)(1) and 1.703(a)(1), the USPTO must now issue a first action within 14 months of commencement of the national stage under section 371(b) or (f), rather than upon fulfillment of the 371 requirements. Since the USPTO's 14-month deadline will start earlier in some national stage applications, this change is beneficial to applicants and may substantially increase PTA in some national stage cases. (2) For patents granted on or after January 14, 2013, the PTA indicated on the patent itself is the USPTO's "official" notification of its PTA determination, and is now the only determination for which an applicant may seek reconsideration under revised Rule 1.705. The deadline to seek such reconsideration is two months from the issue date, extendable by an additional five months pursuant to Rule 1.136(a). The notion of a request for reconsideration of PTA indicated in the notice of allowance has been eliminated. This unitary system will simplify the process of reviewing and petitioning for correction of USPTO-calculated PTA. (3) In its commentary on the Act's revision of 35 USC 154(b)(4)(A), the USPTO set forth its view than an applicant must first seek PTA reconsideration in the USPTO under 35 USC 154(b)(3) prior to filing a civil action under 35 USC 154(b)(4)(A). See 78 FR at 19417 ("A civil action under 35 USC 154(b)(4)(A) is not an alternative to requesting reconsideration of a patent term adjustment under 35 USC 154(b)(3), but is the [exclusive] remedy for an applicant who is dissatisfied with the Director’s decision on the applicant’s request for reconsideration ...."). The USPTO apparently believes this limitation is only effective for patents granted on or after January 14, 2013, see id. at 19418, but it seems possible courts will apply this limitation to all 154(b)(4)(A) civil actions filed on or after January 14, 2013. The effective date of the Interim Final Rule is April 1, 2013. The changes to 37 CFR 1.702, 1.703, and 1.705 apply to patents granted on or after January 14, 2013. The change to 37 CFR 1.704 applies to any application in which a notice of allowance was mailed on or after April 1, 2013. Since the effective date vis-a-vis existing applications and patents is not clearly stated in the AIA Technical Corrections Act, it is possible the USPTO's interpretation of the effective dates set forth above may be the subject of future litigation. Note that the USPTO issued the notice as an Interim Final Rule to allow for public comment by May 31, 2013. Since the revision discussed in item (1) may substantially increase PTA in some national stage cases, applicants are advised to recheck PTA in national stage cases granted as patents on or after January 14, 2013 and seek reconsideration of USPTO-calculated PTA as appropriate. The above description is general in nature, and the PTA effect in an individual patent will depend on the specifics of its prosecution history. Please see the Interim Final Rule and the AIA Technical Corrections Act for complete details of this revision. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
January 29, 2013: In a second Exelixis case, Exelixis, Inc. v. Kappos, No. 1:12cv574 (E.D. Va. 2013) (“Exelixis II”), Judge Brinkema has rejected Judge Ellis’s holding in Exelixis I, which overturned a long-standing USPTO interpretation concerning the 3-Year Application Pendency Guarantee (also called "B Delay" or the "3-Year Issue Guarantee"). See our prior posts below for an overview of the Exelixis I decision. The Exelixis II case involves the same parties as in Exelixis I, but is a separate civil suit seeking PTA correction on a different patent. Since the USPTO has already appealed the Exelixis I decision, this split within the US District Court for the Eastern District of Virginia will likely be resolved by the US Court of Appeals for the Federal Circuit, perhaps later this year. Until this is resolved, to preserve their rights, applicants and patent holders are advised to recheck USPTO-calculated PTA in light of Exelixis I, seeking reconsideration under 37 CFR 1.705, 35 USC 154(b)(4)(A), and other mechanisms as appropriate. Importantly, in considering options for PTA correction, as noted below, HR 6621 signed into law on January 14, 2013, arguably mandates that applicants first seek reconsideration in the USPTO prior to filing a civil action under 35 USC 154(b)(4)(A).
January 15, 2013: [Updated] HR 6621, an act providing technical corrections to the America Invents Act (AIA) and Title 35, was signed into law on January 14, 2013. Among other things, the Act includes several important provisions concerning PTA and may arguably, for some issued patents, retroactively affect an applicant's right to seek PTA correction in US District Court. PTA-related provisions include: (1) The Act revises the USPTO 14-month first action requirement under 35 USC 154(b)(1)(A)(i)(II) for national stage cases. Under the revised provision, the USPTO must issue a first action within 14 months of commencement of the national stage under section 371, rather than upon fulfillment of the 371 requirements. Since the USPTO's 14-month deadline will start earlier in some national stage applications, this change is beneficial to applicants and may substantially increase PTA in some national stage cases. (2) The Act clarifies that the USPTO's 3-year issue deadline under 35 USC 154(b)(1)(B) ("B Delay") begins on "the actual filing date of the application under section 111(a) in the United States or, in the case of an international application, the date of commencement of the national stage under section 371 in the international application." This clarification is seemingly consistent with current USPTO practice in calculating PTA. (3) The Act allows the USPTO to provide its PTA determination at issuance. Under the current bifurcated system, the USPTO provides both a preliminary determination at allowance and a final determination at issuance, each of which must be separately audited by the applicant. See 37 CFR 1.705(b) and (d). Once implemented by the USPTO via regulatory changes, a unitary system will simplify the process of reviewing and petitioning for correction of USPTO-calculated PTA. (4) The Act modifies a patentee's right to seek correction of USPTO-calculated PTA in US District Court under 35 USC 154(b)(4)(A). The revised subsection provides in part: An applicant dissatisfied with the Director’s decision on the applicant’s request for reconsideration under paragraph (3)(B)(ii) shall have exclusive remedy by a civil action against the Director filed in the United States District Court for the Eastern District of Virginia within 180 days after the date of the Director’s decision on the applicant’s request for reconsideration. 35 USC 154(b)(4)(A) (emphasis added). For this subsection, key changes include: - The reference to a "request for reconsideration" (e.g., a petition under 37 CFR 1.705) has been added, arguably mandating that an applicant must first seek PTA reconsideration in the USPTO prior to filing a civil action. For existing patents where no request for reconsideration was filed, the USPTO may argue that a civil remedy is not available.
- The 180-day deadline begins upon the "Director’s decision on the applicant’s request for reconsideration" instead of the issue date, providing some applicants additional time to file a civil action. For applications where the Director's decision comes before issuance, however, less than 180 days from issuance may be available. This revision seems to partially adopt the tolling rule applied in several recent decisions. See, e.g., Bristol-Myers Squibb Co. v. Kappos, 841 F. Supp.2d 238, motion for reconsideration denied, 2012 WL 4127636 (D.D.C. Sept. 20, 2012).
- The 35 USC 154(b)(4)(A) remedy is "exclusive," arguably precluding other legal theories under which applicants might seek PTA correction. See, e.g., Novartis AG v. Kappos, Civil Action No. 10-cv-1138 (Nov. 15, 2012).
The effective date of the above revisions vis-a-vis existing applications and patents is not completely clear and will likely be the subject of litigation. Per the Act, the amendments take effect upon enactment and "shall apply to proceedings commenced on or after such date of enactment." Thus, if the prosecution of a patent application is a "proceeding" that is "commenced" upon filing (or, in a national stage application, upon commencement of the national stage under 35 USC 371), arguably only new patent applications are affected by items (1) to (3) discussed above. Alternatively, perhaps applicants will argue that a 37 CFR 1.705 request for reconsideration, or a civil action under 35 USC 154(b)(4)(A), is a "proceeding," in an attempt to apply item (1) retroactively. For item (4), however, if a civil action under 35 USC 154(b)(4)(A) is a "proceeding" that is "commenced" upon filing a complaint, all prospective civil suits, even those concerning existing patents, may arguably be subject to the revision. The above description is general in nature, and the PTA effect in an individual patent will depend on the specifics of its prosecution history. Please see the Act for complete details. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
January 02, 2013: The USPTO has appealed the ruling in Exelixis, Inc. v. Kappos, No. 1:12cv96, __ F.Supp.2d __ (E.D. Va. 2012) to the United States Court of Appeals for the Federal Circuit. While the appeal is pending, to preserve their rights, applicants and patent owners are strongly advised to recheck USPTO-calculated PTA in light of Exelixis, seeking correction under 37 CFR 1.705, 35 USC 154(b)(4)(A), and other mechanisms as appropriate. Please see our prior posts for more information about Exelixis.
November 12, 2012: The US District Court for the Eastern District of Virginia has overturned a long-standing USPTO interpretation concerning the 3-Year Application Pendency Guarantee (also called "B Delay" or the "3-Year Issue Guarantee") of the American Inventor’s Protection Act ("AIPA"), codified at 35 USC 154(b)(1)(B). See Exelixis, Inc. v. Kappos, No. 1:12cv96 (E.D. Va. 2012). If it stands, this ruling will ensure many more diligent applicants receive a patent term of 17 years or more, as is intended by AIPA's 3-Year Issue Guarantee. One of the patent term guarantees provided by AIPA is that an application must issue within a maximum of three years from filing, or else a day-for-day term extension is made as one component of Patent Term Adjustment ("PTA"). See 35 USC 154(b)(1)(B). Determining how the end of the 3-year issue deadline operates to benefit applicants, however, is not just a simple matter of adding three years to the filing date. Excluded from the 3-year period are specified "tolling events" (also known as "exclusions"), namely (1) time consumed by RCE's; (2) time consumed by interference proceedings, secrecy orders, or appellate review; and (3) delay in USPTO processing requested by the applicant. See 35 USC 154(b)(1)(B)(i)-(iii). At issue in Exelixis is the effect on B Delay of a tolling event, the filing of an RCE, occurring after the 3-year issue deadline has already passed--in other words, after B Delay has already begun to accrue. The long-standing USPTO position is that an RCE always cuts off any subsequent B Delay, even if filed after the end of the 3-year issue deadline. In some long, complex prosecutions with one or more RCE's, this means a relatively small PTA contribution from the 3-Year Issue Guarantee (B Delay), despite the fact that issuance may take a great deal longer than three years. While Exelixis recognizes that "time devoted to an RCE tolls the running of the three year clock if the RCE is filed within the three year period," the Court holds that RCE's filed later do not cut off B Delay: [P]ut simply, RCE's have no impact on PTA if filed after the three year deadline has passed. Exelixis, Slip Op. at 16 (emphasis added). To illustrate this holding, consider a simple prosecution example where a patent is issued seven years from filing, with an RCE filed at Year 4, and no other prosecution events affecting B Delay or other PTA. Under the USPTO interpretation, the RCE cuts off any subsequent B Delay, even though it was filed after the end of the 3-year period: 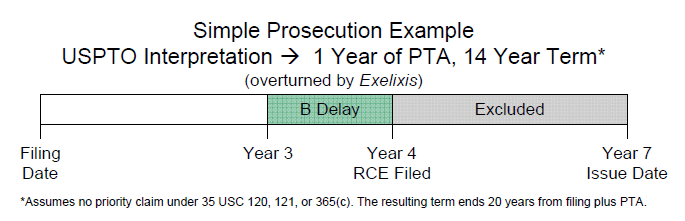
Thus, under the USPTO interpretation, B Delay begins at Year 3, and stops when the RCE is filed at Year 4. Accordingly, only 1 year of PTA accrues. The resulting patent term is 14 years (a 13 year base term plus 1 year of PTA). In contrast, under Exelixis, any RCE filed after the end of the 3-year period has no impact on B Delay: 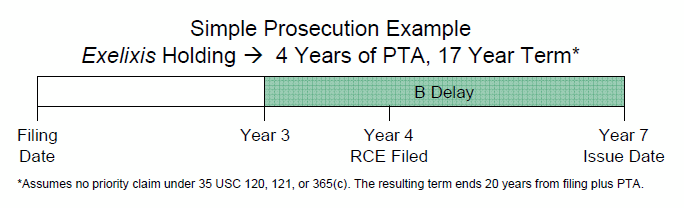
Thus, under Exelixis, B Delay begins at Year 3, and continues until issuance at Year 7. Accordingly, 4 years of PTA accrues. This results in a full 17 year patent term (same 13 year base term plus 4 years of PTA). When prosecuting applications, practitioners are advised to carefully consider the timing of tolling events (e.g., an RCE or appeal) vis-a-vis the effective end of the 3-year period in 35 USC 154(b)(1)(B) since, under Exelixis, a few days difference can have a major impact on the term of a resulting patent. For example, a first RCE filed at two years and eleven months from the application filing date acts to toll the 3-year issue deadline, whereas the same RCE filed at three years and a day (or, if other tolling events exist, after whatever date effectively ends the 3-year deadline) has no impact on B Delay. In other words, from an applicant perspective, it is now even more important that B Delay actually begins since, under Exelixis, once started it will run continuously until issuance, providing very significant term protection against further prosecution delays. For existing applications and patents, applicants and patent owners are advised to recheck USPTO-calculated PTA in light of Exelixis, seeking correction under 37 CFR 1.705, 35 USC 154(b)(4)(A), and other mechanisms as appropriate. We believe it is likely the USPTO will appeal the Exelixis decision, so its full impact may not be known for some time. The USPTO may also seek to define the filing of some or all RCE's as applicant delay per 35 USC 154(b)(2)(C)(iii) (i.e., a circumstance that constitutes a failure of an applicant to engage in reasonable efforts to conclude processing or examination of an application), although such a characterization may be farfetched. And, any new USPTO interpretation or regulation seeking to limit Exelixis would likely be complex to account for overlaps with other USPTO and applicant delays. The above description is general in nature, and the PTA effect in an individual patent will depend on the specifics of its prosecution history. Please see the Exelixis decision for complete details. If you need assistance or have questions about performing a PTA calculation in accordance with Exelixis, please contact us via e-mail to Support@PatentTerm.com.
August 22, 2012: The USPTO has issued a Final Rule revising the following PTA provisions relating to Board appeals: (1) PTA Credit for Successful Appellate Review. Under 35 USC 154(b)(1)(C)(iii), applicants receive PTA for the pendency of appellate review, by the Board or a Federal court, if the appeal is successful (i.e., patent issues under a decision reversing an adverse determination of patentability). By this rule change, the USPTO has redefined the pendency of an appeal to start when jurisdiction passes to the Board, instead of upon the filing of a notice of appeal by the applicant. Compare pre-revision 37 CFR 1.703(e) with post-revision 37 CFR 1.703(e). (2) Exclusion for Appellate Review. Under 35 USC 154(b)(1)(B)(ii), any time consumed by appellate review, by the Board or a Federal court, is not included in determining if the USPTO failed to issue a patent within 3 years under 35 USC 154(b)(1)(B). For this exclusion, the USPTO has redefined the time consumed by an appeal to start when jurisdiction passes to the Board, instead of upon the filing a notice of appeal by the applicant. Compare pre-revision 37 CFR 1.703(b)(4) with post-revision 37 CFR 1.703(b)(4). Accordingly, if jurisdiction does not pass to the Board (e.g., examiner issues a new Office action or allowance before briefing), there is no exclusion under 35 USC 154(b)(1)(B)(ii). (3) 3-Month Applicant Deadline to File Appeal Brief. The USPTO has established a new PTA rule requiring applicants to file a compliant appeal brief or RCE within 3 months of filing a notice of appeal. If this time limit is exceeded, it is considered applicant delay, and PTA credits are reduced. See revised 37 CFR 1.704(c)(11). The Final Rule is effective September 17, 2012. Changes to Rule 1.703 apply to any application in which a notice of allowance is issued on or after September 17, 2012, and any patent issuing thereon. The change to Rule 1.704 applies with respect to the filing of an appeal brief in any application in which a notice of appeal under 37 CFR 41.31 is filed on or after September 17, 2012. In addition, the USPTO will also apply the changes to Rule 1.703 in any timely PTA reconsideration proceeding initiated on or after September 17, 2012. To allow patentees to take advantage of the rule change to potentially increase PTA, the Notice suggests several avenues to seek reconsideration, including filing a request for reconsideration within two months of a USPTO PTA decision under 37 CFR 1.705(d). The above description is general in nature, and the PTA effect in an individual patent will depend on the specifics of its prosecution history. Please see the Final Rule for complete details of this revision. If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com.
December 04, 2011: The USPTO has issued a Final Rule expanding the 37 CFR 1.704(d) safe harbor provision exempting certain Information Disclosure Statement (IDS) filings from PTA reduction under 37 CFR 1.704(c)(6), (8), (9), or (10). This change will increase patent term for many patentees, especially in complex cases where applicants are required to file numerous IDS's disclosing actions or references in related applications. To benefit, however, applicants will need to file IDS's within 30 days of pertinent US, foreign, or international office communications. Under the revised provision, 37 CFR 1.704(d) now exempts IDS's containing only: (1) items first cited in any communication from a patent office in a counterpart foreign or international application or from the USPTO. Previously, only items from communications from a foreign patent office in a counterpart application were explicitly covered. (2) communications issued by a patent office in a counterpart foreign or international application or by the USPTO. Previously, only items cited in communications, as opposed to communications themselves, were explicitly covered. As before, an exempted paper must contain only a compliant IDS, and must be accompanied by the required statement that, inter alia, pertinent communications were not received by any individual designated in § 1.56(c) more than 30 days prior to the IDS filing. See 37 CFR 1.704(d) for complete requirements.
August 23, 2011: We have added an option to analyze the 3-Month Applicant Response rule under ArQule, Inc. v. Kappos, No. 10-1904 (D.D.C. 2011). For important information concerning this update, please see the Announcements when you logon to your account, or click the Arqule v. Kappos link, which appears under each occurrence of the 3-Month Applicant Response rule on the Apply Term Rules tab. If you need assistance or have questions concerning this option, please contact us via e-mail to Support@PatentTerm.com.
August 08, 2011: On June 23, 2011, the DC District Court overturned the USPTO's long-standing interpretation that, for PTA purposes, weekend and holiday exceptions under 35 USC 21(b) do not apply to the 3-month applicant response deadline under 35 USC 154(b)(2)(C)(ii). See ArQule, Inc. v. Kappos, No. 10-1904 (D.D.C. 2011). In ArQule, a 3-month applicant response deadline fell on Veterans Day, and the applicant filed its response the following day. The district court held that the USPTO’s one day reduction of applicant’s PTA was improper since, under the clear meaning of the statute, the weekend and holiday exception in 35 USC 21(b) does apply when calculating PTA reduction under 35 USC 154(b)(2)(C)(ii). If this ruling stands, it is likely to increase the PTA for thousands of applications, albeit by a small number of days. As of early August, to our knowledge, the USPTO has not yet appealed the DC District Court judgment, nor has the USPTO issued a notice concerning the ruling. Applicants are advised to carefully check USPTO-calculated PTA for consistency with ArQule, taking into account the deadlines for seeking correction under 37 CFR 1.705 and 35 USC 154(b)(4). To perform such calculations using the PatentTerm Online calculator, where a weekend or holiday affects PTA under the 3-month applicant response deadline, users will need to manually adjust pertinent dates to account for weekends and holidays. Keep in mind that a separate response event may need to be added, since the 35 USC 21(b) exception presumably does not affect the 4-month USPTO deadline to respond to certain applicant papers. See 35 USC 154(b)(1)(A)(ii); 37 CFR 1.702(a)(2), 1.703(a)(2),(3). If you need assistance or have questions, please contact us via e-mail to Support@PatentTerm.com. In the next few weeks, PatentTerm Online will implement an update allowing a more automated solution addressing this issue.
August 29, 2010: The USPTO has implemented a revised PAIR Patent Term Adjustment tab showing several new details of its PTA calculation. New fields include separate totals for "A," "B," and "C" USPTO delays under 35 USC 154(b)(1), and overlaps therebetween. Major changes are highlighted below:  This PAIR system update was outlined in the Federal Register Notice of February 1, 2010, and an explanation by the USPTO is available here.
July 21, 2010: The USPTO has issued a Federal Register Notice indicating it will no longer review Duty of Candor and Good Faith Letters. Instead, such letters will simply be placed in the file wrapper without further review, and certificates of correction will not be issued based on such letters. Historically, applicants have used Duty of Candor and Good Faith Letters to disclose where the USPTO has granted PTA greater than what the applicant believes is appropriate. The USPTO would typically review its PTA calculation in light of such letters and make corrections as warranted. Now, even where the USPTO errs in granting excessive PTA, the applicant must use the procedures in 37 CFR 1.705 to obtain USPTO reconsideration of a PTA determination. This includes satisfying the formal requirements and deadlines set forth in Rule 1.705 and paying a $200 fee. Alternatively, a patentee may file a terminal disclaimer disclaiming the period considered in excess of the appropriate PTA, but presumably this will not invoke substantive PTA reconsideration by the USPTO. The USPTO did not provide an opportunity for comments prior to this Notice, and did not explain the effect of unreviewed letters. The USPTO indicated it will revise appropriate MPEP sections accordingly in due course. Please review the Federal Register Notice for details.
July 13, 2010: On July 6th, Novartis filed suit against the USPTO seeking PTA correction under Wyeth for eleven US patents. Of note, since the subject patents issued as early as December 2003, Novartis is seeking to retroactively apply Wyeth well beyond the 180 day deadline set forth in 35 USC 154(b)(4) and the USPTO Interim Procedure to Request PTA Recalculation. Novartis alleges, inter alia: - For older patents, the USPTO does not provide an administrative remedy to recapture PTA deprived under its flawed overlap interpretation overturned in Wyeth. The USPTO's refusal to accord the benefits of Wyeth to older patents is "arbitrary, capricious, an abuse of discretion, or otherwise not in accordance with law and in excess of statutory jurisdiction, authority or limitation."
- The 180 day deadline set forth in 35 USC 154(b)(4) applies only to PTA determinations made in conjunction with a notice of allowance, not PTA determinations made at issuance.
- Even if the 35 USC 154(b)(4) deadline applies to Novartis' claims, it is tolled under the common law doctrine of equitable tolling. In that case, the 180 day period would begin on January 7, 2010 (Federal Circuit affirms Wyeth) or January 21, 2010 (USPTO announces it will not appeal Wyeth).
- The USPTO's "purposeful and deliberate diminution of the patent term ... constitutes a taking of Novartis' property without just compensation, in violation of the Fifth Amendment of the Constitution of the United States."
January 29, 2010: The USPTO announced the following interim procedure to request PTA recalculation under Wyeth: - Patentees can request PTA recalculation under Wyeth without a fee or petition.
- The procedure is only available for patents issued prior to March 2, 2010.
- A recalculation request must be filed no later than 180 days after the patent issue date.
- The interim procedure is only available if the sole basis for alleged error is the Wyeth issue.
- Form PTO/SB/131 has been made available to make the recalculation request.
- The USPTO has submitted a Federal Register Notice setting forth details.
One leading commentator, however, has questioned the USPTO's authority to provide this interim procedure. Prudent applicants are advised to recheck all parts of USPTO-calculated PTA, observing the formal deadlines under 35 USC 154(b)(4) and 37 CFR 1.705 where possible. Otherwise, applicants may have limited recourse to correct erroneous USPTO recalculations, especially if the recalculation is made more than 180 days after issue.
January 22, 2010: The USPTO has announced that it, and the Department of Justice, have decided not to seek further review of the Federal Circuit's decision in Wyeth v. Kappos. The USPTO further stated it is preparing guidance for expediting requests for PTA recalculation in light of Wyeth, which will be issued as soon as possible. The USPTO again reminds applicants and patent owners dissatisfied with a USPTO-calculated PTA of "the requirement to seek review of that determination within 180 days of patent issuance and the time periods set in the implementing regulations. See 35 USC sec. 154(b)(4) and 37 CFR 1.705." For patents outside the listed deadlines, perhaps some practitioner will succeed in retroactively obtaining the benefit of Wyeth (e.g., certificate of correction, petition to director, equitable tolling), but that path is less certain.
January 12, 2010: While not yet implemented, the USPTO has announced it is in the process of changing its PTA calculation algorithm to conform with Wyeth, pending a determination by the Solicitor General of whether to seek further review of the Federal Circuit holding. Until the change is implemented and tested, Applicants are advised to carefully check USPTO-calculated PTA under Wyeth. The USPTO reminds applicants and patent owners dissatisfied with a USPTO-calculated PTA of "the requirement to seek review of that determination within 180 days of patent issuance and the time periods set in the implementing regulations. See 35 USC 154(b)(4) and 37 CFR 1.705." For patents outside the listed deadlines, perhaps some practitioner will succeed in retroactively obtaining the benefit of Wyeth (e.g., certificate of correction, petition to director, equitable tolling), but that path is less certain.
January 12, 2010: PatentTerm Online has issued a press release briefly explaining, in layperson's terms, the history and holding on the issue presented in Wyeth. See Inventors Take Heart: Federal Court Restores Patent Term Guarantees, at Last Protecting Innovators from Patent Office Delays, Noted Professor Says.
January 07, 2010: [Updated] Today, the Federal Circuit unanimously affirmed the DC District Court ruling in Wyeth, overturning a USPTO statutory interpretation that resulted in reduced terms for tens of thousands of US patents. This holding, long advocated by the founders of PatentTerm Online, LLC, will result in significantly greater PTA for many patent applications pending more than three years. Click here to download the Wyeth v. Kappos opinion. Applicants are advised to carefully check USPTO-calculated PTA to ensure the full grant of proper adjustment credits under Wyeth. By default, our calculator will automatically apply the Wyeth holding in calculating PTA. (Or, optionally, the calculator can also apply the pre-Wyeth USPTO interpretation.) To learn more about the PTA benefits of Wyeth, please review our updated simplified example.
August 24, 2009: [Wyeth Briefing Update] Briefing was completed in Wyeth v. Kappos, No. 09-1120 (Fed. Cir.), on June 22, 2009. Oral argument has been scheduled for October 7, 2009.
June 05, 2009: We have confirmed that the USPTO is rejecting some Applications for PTA requesting additional days under the Wyeth ruling. To seek review of a USPTO PTA determination, applicants must file a civil action in the DC District Court within 180 days after the grant of the patent. See 35 USC 154(b)(4)(A). This deadline may apply even where the USPTO has not acted on a pending PTA petition.
November 28, 2008: The USPTO appealed the ruling in Wyeth et al. v. Dudas, 580 F.Supp.2d 138 (D.D.C. 2008). The initial appeal was to the DC Circuit Court, but was subsequently transferred to the Federal Circuit.
November 25, 2008: On November 7, 2008, Ironwood Pharmaceuticals filed suit against the USPTO seeking additional PTA under Wyeth. Of note, the applicant proceeded directly to the DC District Court within 180 days of issue under 35 USC 154(b)(4)(A) without first seeking correction in the USPTO under 37 CFR 1.705.
October 02, 2008: There has been a significant development in PTA law. On September 30, 2008, the DC District Court overturned the USPTO's interpretation of 35 USC 154(b)(2)(A) (the "Actual Delay" limitation) in Wyeth et al. v. Dudas, 580 F.Supp.2d 138 (D.D.C. 2008).
Disclaimer: News, articles, commentary, examples, and authorities available here are provided on an "as is" and "as available" basis, are not legal advice, and my contain errors or omissions, or may no longer be correct under current law." Third-party sites will appear in a separate browser window and are not affiliated with PatentTerm Online, LLC. Links to third-party sites are provided solely for your convenience and do not constitute endorsement by PatentTerm Online, LLC.
|